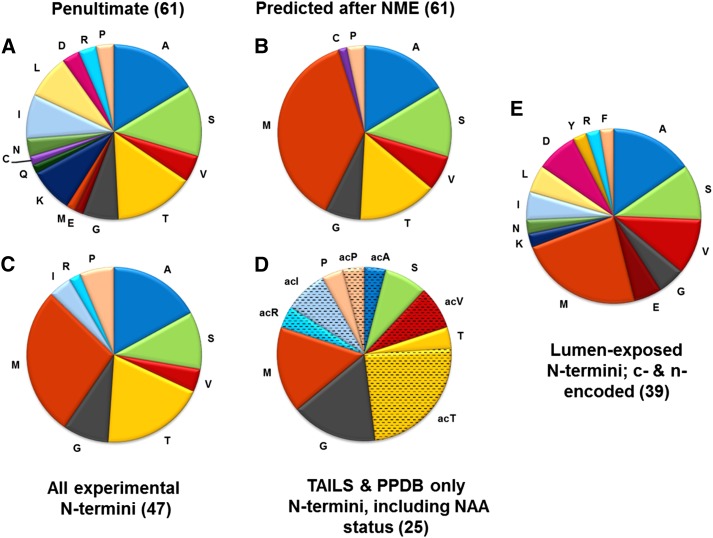
Figure 4.
Nt amino acid frequency for stroma-exposed p-encoded proteins and comparison with all known lumenally exposed Nti (both p-encoded and n-encoded proteins). Detailed information is available in Supplemental Table S6. A, The penultimate residues (i.e. residues immediately downstream of the initiating Met) of 65 p-encoded proteins for which the N terminus is facing the stroma. This sequence information is derived from the protein sequences listed in The Arabidopsis Information Resource (TAIR; https://www.arabidopsis.org/). Within this group, there are three sets of identical homologs (ribosomal proteins S7A,B, ribosomal proteins S12A,B,C, and a full-length YCF1.2 protein and a truncated form; for details, see Supplemental Table S6). Rather than including each of these homologs, we counted each set only once, thus resulting into 61 Nti. B, The predicted Nt residues of mature proteins after application of the general NME rule for the p-encoded proteins in A. C, Experimentally determined Nt residues for p-encoded proteins for which the N terminus is facing the stroma (a total of 47 proteins). Experimental evidence was obtained from the TAILS experiments described in this study, from semitryptic or NAA Nti detected previously (Zybailov et al., 2008, 2009; Bienvenut et al., 2012), and additional data from in-house experiments in PPDB. Also included is information from Giglione et al. (2004), which were mostly based on Nt Edman sequencing data from various plant species. We note that Edman sequencing cannot sequence proteins for which the Nt is NAA; these modified Nti are blocked, preventing Edman chemistry. The experimental Nt information from these other plant species was projected onto Arabidopsis homologs if the Nti were identical. D, Experimentally determined Nt residues for 25 p-encoded proteins for which the N terminus is facing the stroma as determined by TAILS and in-house experiments in PPDB. This is a subset of the proteins in C. E, Experimentally determined Nt residues for 39 p-encoded and n-encoded proteins for which the N terminus is facing the thylakoid lumen. Experimental evidence was obtained from the TAILS experiments, previous publications (Zybailov et al., 2008, 2009), and additional data in PPDB (for details, see Supplemental Table S7).