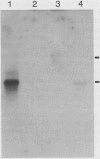
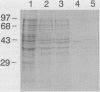
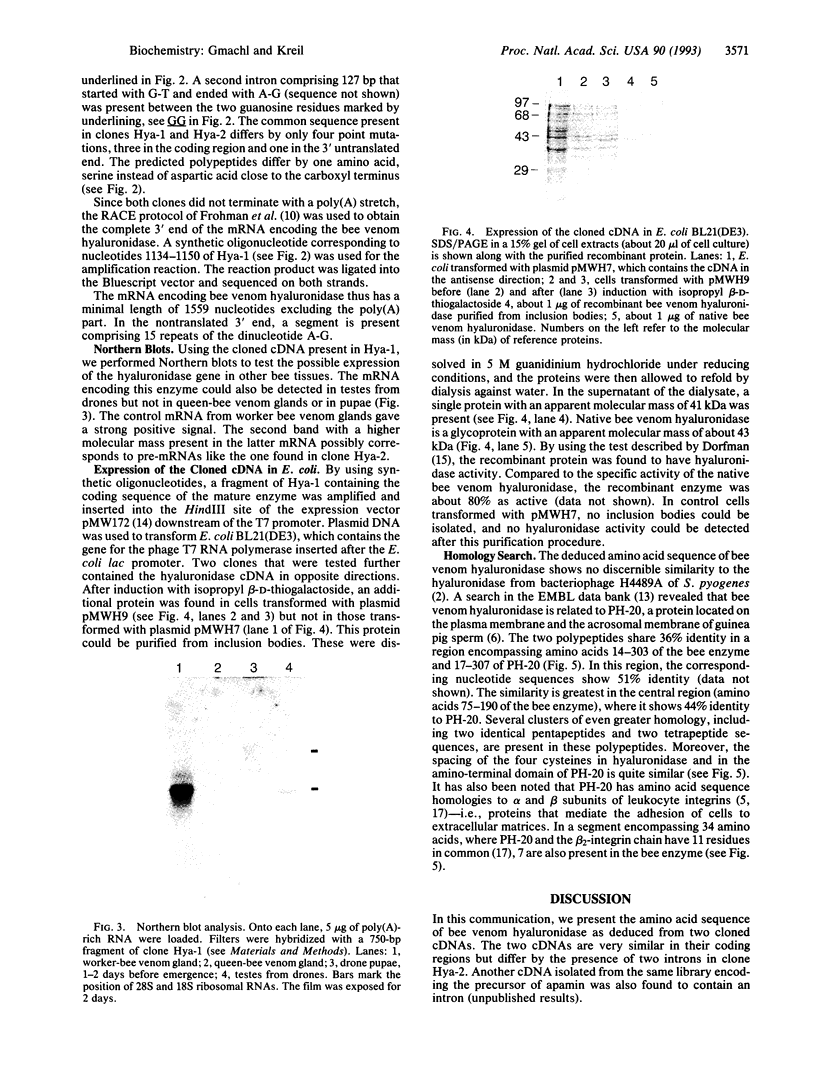
Abstract
The venom of honeybees, Apis mellifera, contains several biologically active peptides and two enzymes, one of which is a hyaluronidase. By using degenerate oligonucleotides derived from the amino-terminal sequence of this hyaluronidase reported by others, clones encoding the precursor for this enzyme could be isolated from a cDNA library prepared from venom glands of worker bees. The deduced amino acid sequence showed that bee venom hyaluronidase is a polypeptide composed of 349 amino acids containing four cysteines and three potential sites for N-glycosylation. The sequence of the precursor also indicated that the conversion of the pro-enzyme to the end product must involve cleavage of a Thr-Pro bond, a most unusual processing reaction. The mRNA encoding hyaluronidase could also be detected in testes from drones. Expression of the cloned cDNA in Escherichia coli yielded a 41-kDa polypeptide that had hyaluronidase activity. Interestingly, the hyaluronidase from bee venom glands exhibited significant homology to PH-20, a membrane protein of guinea pig sperm involved in sperm-egg adhesion. These structural data support the long-held view that hyaluronidases play a role in fertilization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eppig J. J. FSH stimulates hyaluronic acid synthesis by oocyte-cumulus cell complexes from mouse preovulatory follicles. Nature. 1979 Oct 11;281(5731):483–484. doi: 10.1038/281483a0. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. A single-chain integrin? New Biol. 1991 May;3(5):525–526. [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hardwick C., Hoare K., Owens R., Hohn H. P., Hook M., Moore D., Cripps V., Austen L., Nance D. M., Turley E. A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992 Jun;117(6):1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hynes W. L., Ferretti J. J. Sequence analysis and expression in Escherichia coli of the hyaluronidase gene of Streptococcus pyogenes bacteriophage H4489A. Infect Immun. 1989 Feb;57(2):533–539. doi: 10.1128/iai.57.2.533-539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Dalton N., Lawrence A. J., Pearce F. L., Vernon C. A. The purification and characterisation of hyaluronidase from the venom of the honey bee, Apis mellifera. Eur J Biochem. 1984 Mar 1;139(2):217–223. doi: 10.1111/j.1432-1033.1984.tb07997.x. [DOI] [PubMed] [Google Scholar]
- Kreil G., Haiml L., Suchanek G. Stepwise cleavage of the pro part of promelittin by dipeptidylpeptidase IV. Evidence for a new type of precursor--product conversion. Eur J Biochem. 1980 Oct;111(1):49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Gmachl M., Sippl M. J., Kreil G. Analysis of the cDNA for phospholipase A2 from honeybee venom glands. The deduced amino acid sequence reveals homology to the corresponding vertebrate enzymes. Eur J Biochem. 1989 Sep 1;184(1):249–254. doi: 10.1111/j.1432-1033.1989.tb15014.x. [DOI] [PubMed] [Google Scholar]
- Lathrop W. F., Carmichael E. P., Myles D. G., Primakoff P. cDNA cloning reveals the molecular structure of a sperm surface protein, PH-20, involved in sperm-egg adhesion and the wide distribution of its gene among mammals. J Cell Biol. 1990 Dec;111(6 Pt 2):2939–2949. doi: 10.1083/jcb.111.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. J., Macek M. B., Shur B. D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature. 1992 Jun 18;357(6379):589–593. doi: 10.1038/357589a0. [DOI] [PubMed] [Google Scholar]
- Owen M. D. Relationship between age and hyaluronidase activity in the venom of queen and worker honey bees (Apis mellifera L.). Toxicon. 1979;17(1):94–98. doi: 10.1016/0041-0101(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps B. M., Primakoff P., Koppel D. E., Low M. G., Myles D. G. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science. 1988 Jun 24;240(4860):1780–1782. doi: 10.1126/science.3381102. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Lathrop W., Woolman L., Cowan A., Myles D. Fully effective contraception in male and female guinea pigs immunized with the sperm protein PH-20. Nature. 1988 Oct 6;335(6190):543–546. doi: 10.1038/335543a0. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Myles D. G. A map of the guinea pig sperm surface constructed with monoclonal antibodies. Dev Biol. 1983 Aug;98(2):417–428. doi: 10.1016/0012-1606(83)90371-8. [DOI] [PubMed] [Google Scholar]
- Salustri A., Yanagishita M., Hascall V. C. Synthesis and accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell-oocyte complex during follicle-stimulating hormone-induced mucification. J Biol Chem. 1989 Aug 15;264(23):13840–13847. [PubMed] [Google Scholar]
- Salustri A., Yanagishita M., Underhill C. B., Laurent T. C., Hascall V. C. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol. 1992 Jun;151(2):541–551. doi: 10.1016/0012-1606(92)90192-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. Hyaluronidase dissolves a component in the hamster zona pellucida. J Exp Zool. 1984 Feb;229(2):309–316. doi: 10.1002/jez.1402290216. [DOI] [PubMed] [Google Scholar]
- Vlasak R., Kreil G. Nucleotide sequence of cloned cDNAs coding for preprosecapin, a major product of queen-bee venom glands. Eur J Biochem. 1984 Dec 3;145(2):279–282. doi: 10.1111/j.1432-1033.1984.tb08549.x. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Profile of a mammalian sperm receptor. Development. 1990 Jan;108(1):1–17. doi: 10.1242/dev.108.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Way M., Pope B., Gooch J., Hawkins M., Weeds A. G. Identification of a region in segment 1 of gelsolin critical for actin binding. EMBO J. 1990 Dec;9(12):4103–4109. doi: 10.1002/j.1460-2075.1990.tb07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]