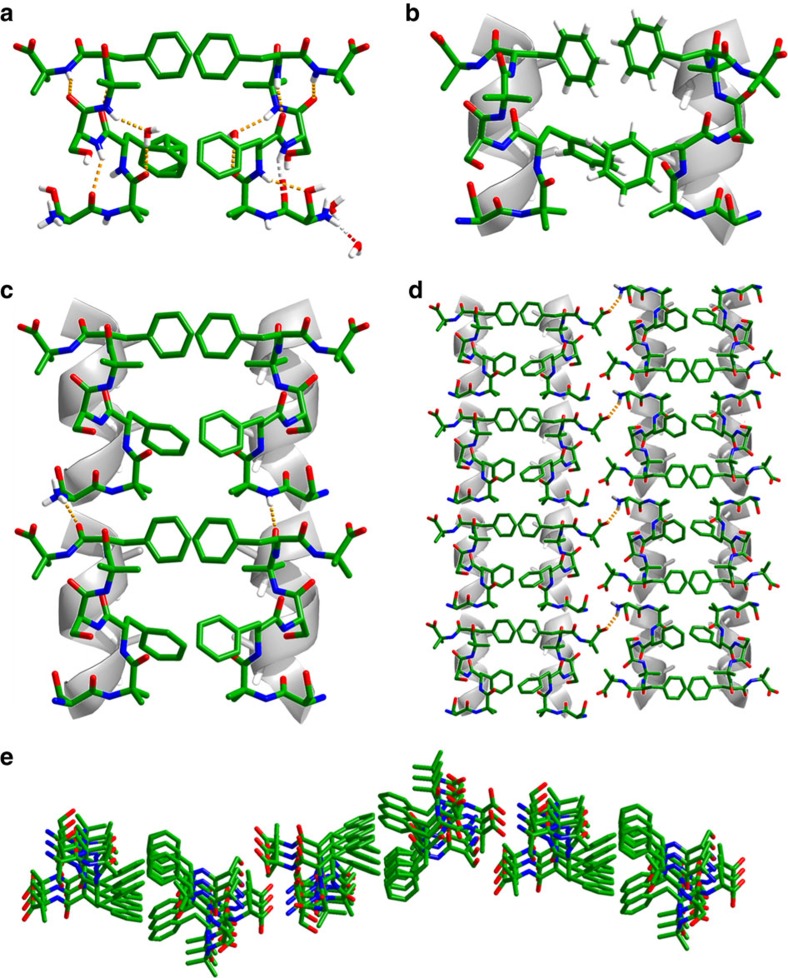
Figure 3. Single crystal X-ray analysis of SHR-FF.
(a) Two different conformations of the molecules present in the asymmetric units. (b) Aromatic–aromatic interactions stabilized the dimeric interface of the conformers. (c) Molecular packing of the peptide creates zipper-like structures in which hydrogen bonds (shown by yellow dotted lines) connect the individual dimers. (d) Antiparallel packing of adjacent zipper modules. (e) View of three adjacent zipper-like structures from the C-terminus of the SHR-FF super-helical organization.