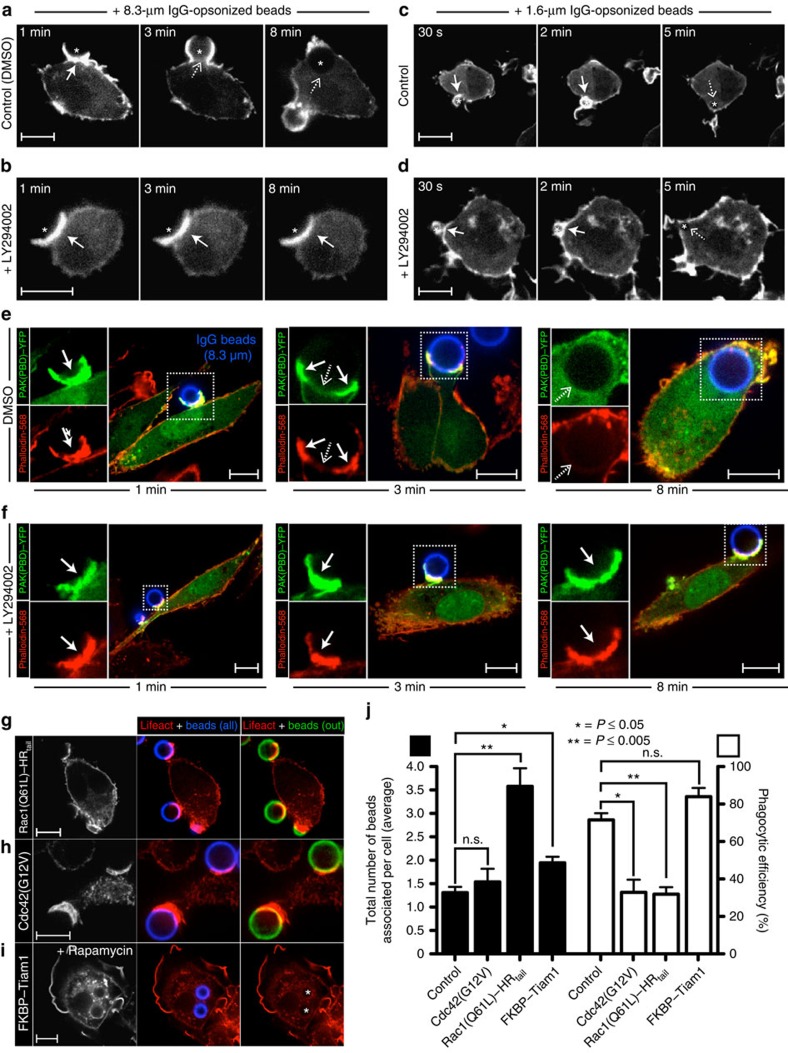
Figure 1. PI3K controls actin disassembly during phagocytosis of large targets.
(a–d) Time-lapse confocal micrographs of RAW 264.7 macrophages transiently expressing Lifeact–mRFP and challenged with 8.3-μm (a,b) or 1.6-μm (c,d) IgG-opsonized beads (signalled with a star). Cells were treated with vehicle (DMSO) or the PI3K inhibitor LY294002 for 10 min before initiating phagocytosis. Actin dynamics were followed throughout the course of engulfment, with the 0-min time point corresponding to the initial engagement of the beads. Solid and dashed arrows point to sites of F-actin accumulation and clearance, respectively. (e,f) Confocal micrographs of primary human macrophages transfected with PAK(PBD)–YFP, a biosensor for active Rac/Cdc42, during phagocytosis of 8.3 μm IgG beads. Before phagocytosis, cells were treated with DMSO (e) or LY294002 (f) for 10 min. Phagocytosis was allowed to proceed for the indicated times, before fixing and staining F-actin with phalloidin. Insets (boxed regions) show magnified views of the phagocytic cup. (g–i) Confocal micrographs of RAW 264.7 macrophages transiently co-expressing Lifeact–mRFP in combination with constitutively active Rac1 (g), constitutively active Cdc42 (h) or a recruitable form of the Rac1 GEF Tiam1 (i). Addition of rapamycin triggers translocation of Tiam1 to the plasmalemma, where its rapamycin-binding domain (FKBP) interacts with a second, complementary rapamycin-binding moiety. Transfectants were challenged with 8.3-μm IgG beads, and phagocytosis allowed to proceed for 10 min before fixation. All phagocytic targets were stained with Cy5-conjugated secondary antibody (shown in blue) before phagocytosis. Extracellular beads were identified by staining fixed (non-permeabilized) cells with an Alexa Fluor 488-conjugated secondary antibody (shown in green). Internalized beads are indicated with a star. Scale bar, 10 μm. (j) Quantification of phagocytic indices (total number of beads associated per cell; black bars) and phagocytic efficiencies (ratio of internalized-to-total number of beads per cell; white bars) for the experiments described in (g–i). Values represent the means of three independent replicates±s.e.m. At least 25 cells were assessed per replicate. *P≤0.05, **P≤0.005 or n.s. (not significant) using Student's two-tailed unpaired t-tests.