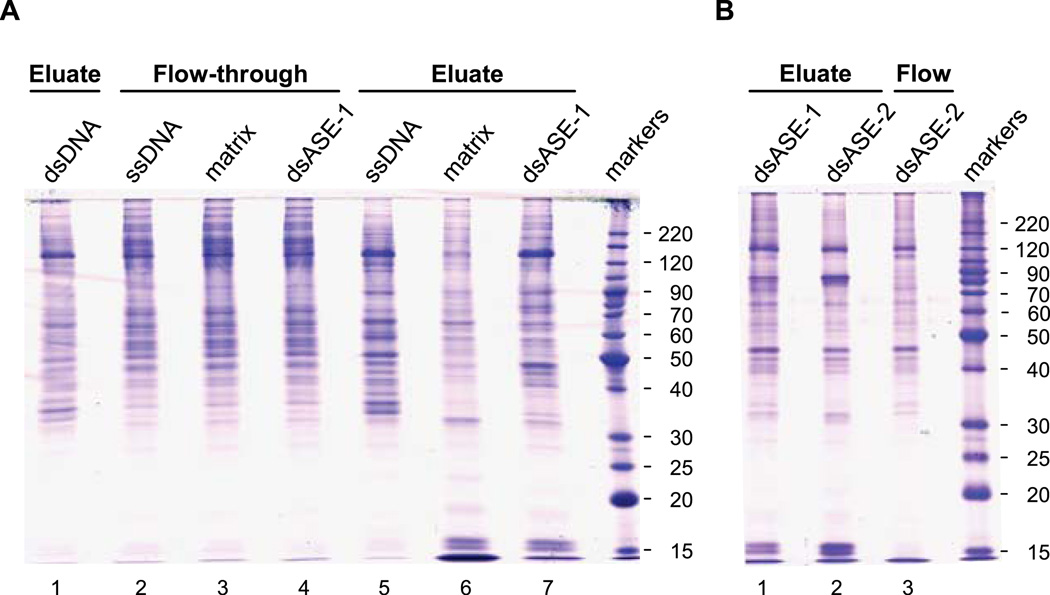
Fig. 3.
SDS-PAGE gels of DNA affinity purification fractions. Proteins from the indicated chromatographic fractions were resolved on an 11% SDS-PAGE gel and visualized by staining with Coomassie Blue. The gel in panel A contained a total of 5 µg of nuclear proteins per lane, while the gel in panel B contained only 3 µg/lane. The gels were placed on a clean plastic plate, and individual lanes cut sequentially into (3-mm) gel slices across their entire length. Proteins within the gel slices were digested (in-gel) with trypsin and the eluted peptides analyzed by mass spectrometry.