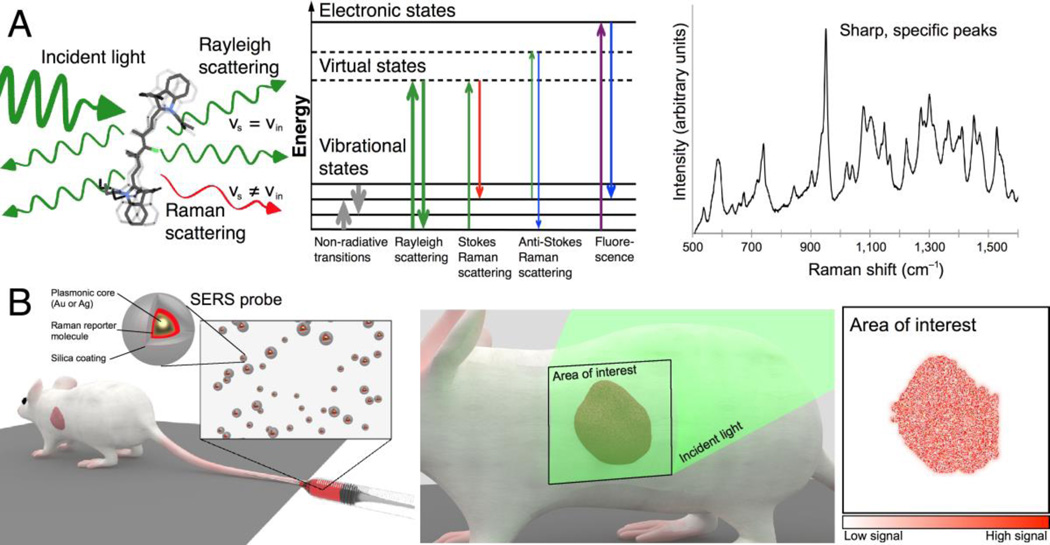
Figure 1.
Principle of surface-enhanced Raman scattering nanoparticles for in vivo cancer detection. (A) Left: Light scattering from a molecule includes both Rayleigh and Raman scattered photons. Middle: Schematic depiction of photon energy transitions during different types of light scattering. Rayleigh scattering is by far the most common form of light scattering, with only few photons undergoing Raman related transitions. Right: Raman spectra exhibit peaks specific to the molecular bond vibrations. Sharp, high-intensity peaks are characteristic of SERS probes. (B) SERS probes can be engineered to create strong SERS signals detectable in vivo. While there are different routes of administration depending on the tumor location and type, in most cases intravenous injection (left) will be the most desirable route. Left inset: SERS probes typically consist of a noble metal core (gold or silver) that provides signal intensity enhancement via surface plasmon resonance effects, a layer of a Raman reporter molecule that gives a specific spectrum, and a passivation layer. Middle: To enable cancer detection, SERS probes must accumulate in cancerous tissue, where they can be detected by their spectral signature upon interrogation with a Raman imaging system. Right: By color-coding the pixels in an acquired image where the unique SERS spectrum of the probe is detected, an image of the tumor is generated.