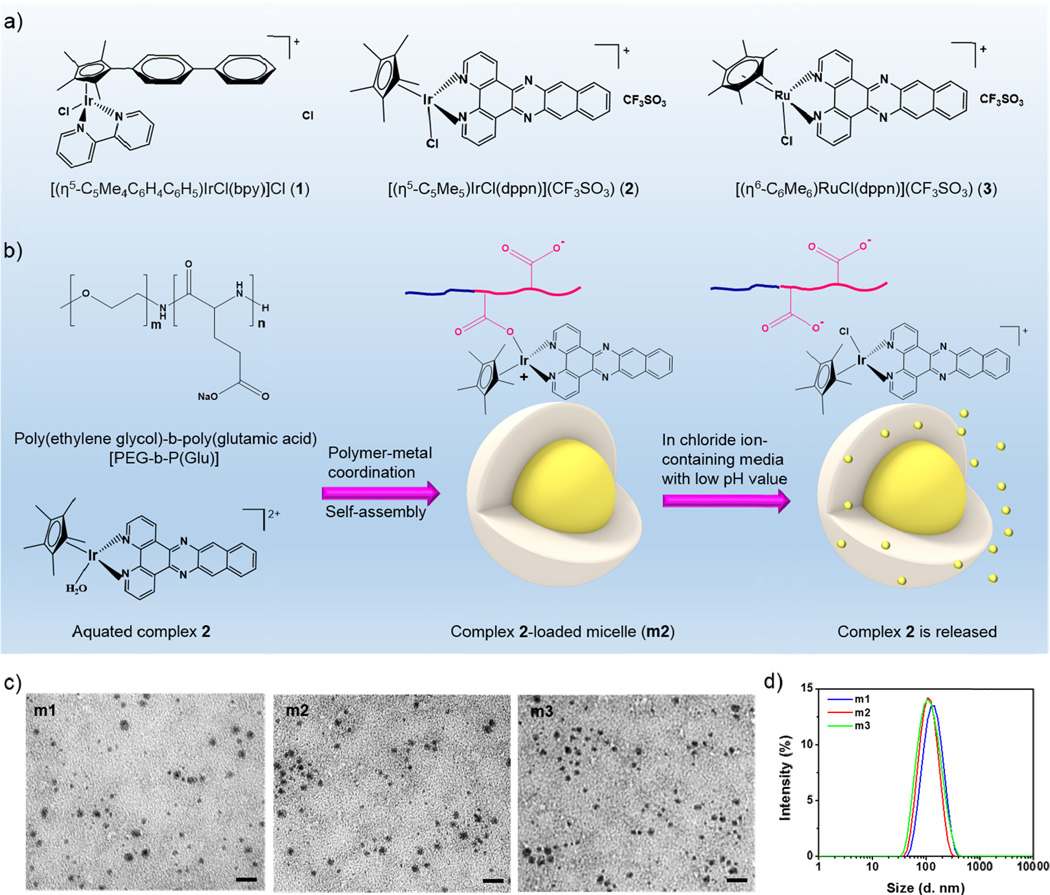
Figure 1.
a) Chemical structures of complexes 1–3. b) Scheme showing the formation of complex-loaded micelles (m1–m3) and the proposed release of the complex from micelles in chloride ion-containing or/and low pH media. The micelles are spontaneously formed via a ligand exchange reaction of metal from the chloride to the carboxylates in the copolymer, and micelle dissociation is accompanied by the release of complex via an inverse ligand exchange reaction of metal from the carboxylates in the copolymer to the chloride in the surrounding media. c) TEM images of micelles showing the well-defined and spherical morphology. Scale bars: 100 nm. d) Size distribution of micelles as determined by DLS.