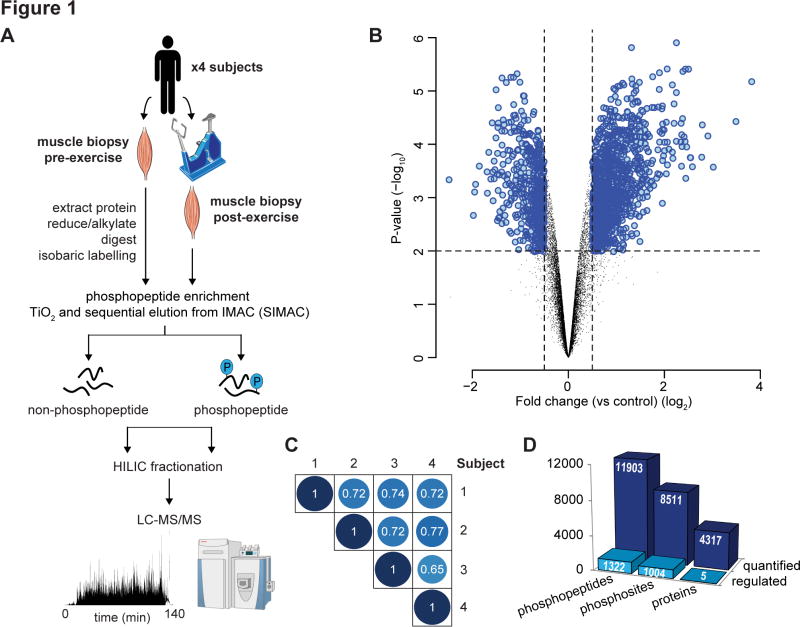
Fig. 1. Acute exercise-regulated phosphoproteome in human skeletal muscle.
(A) Experimental design of the phosphoproteomic analysis of exercise in human muscle is shown. Muscle biopsies pre- and post-exercise from four healthy males were collected. Protein was extracted, digested with Lys-C/trypsin and peptides were isobarically labeled with iTRAQ or TMT tags. Phosphopeptides were enriched by titanium dioxide chromatography and sequential elution from immobilized metal ion affinity chromatography (SIMAC). The unbound non-phosphorylated fraction and phosphorylated fraction was further separated by hydrophilic interaction liquid chromatography (HILIC) into 12–16 fractions. Each fraction was analyzed by nano-ultra high pressure liquid chromatography coupled to tandem MS (nanoUHPLC-MS/MS) on a Q-Exactive MS operated in DDA. (B) Volcano plot showing the median phosphopeptide Log2 fold-change (post- / pre-exercise) plotted against the −Log10 P-value highlighting significantly regulated phosphopeptides (blue; P < 0.05, n=4, moderated t-test). Dotted lines indicate (+/−) 1.5-fold change (Log2 = 0.58). (C) Pearson’s correlation analysis of phosphopeptide fold-change quantification (post- / pre-exercise) between the 4 subjects is shown. (D) Summary of the quantified and regulated proteome and phosphoproteome is shown.