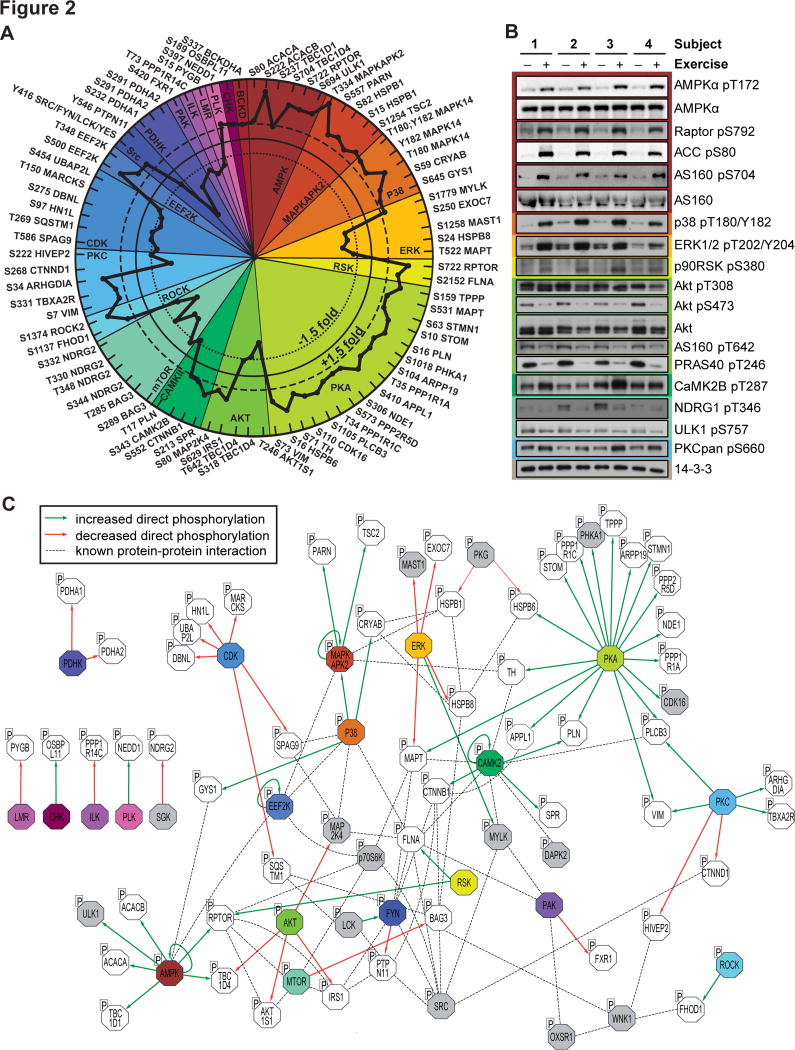
Fig. 2. Site-specific kinase-substrate regulation in response to acute exercise.
(A) Significantly regulated phosphopeptides (+/− 1.5-fold change, P < 0.05, n=4, moderated t-test) were clustered according to PhosphoSitePlus-annotated upstream kinases. Co-regulation of substrate phosphorylation sites (inner dotted circle = decreased phosphorylation; outer dotted circle = increased phosphorylation) provides insights into kinase activity in response to exercise. (B) Muscle biopsy lysates from four subjects pre- and post-exercise were immunoblotted for kinases and substrates in the phosphoproteomics data. (C) An integrative network of the exercise-regulated kinase interactome was generated using experimentally validated human protein-protein interactions and annotated kinase-substrate relationships. Direct increases (green arrows) and decreases (red arrows) in substrate phosphorylation are shown, and dotted lines represent protein-protein interactions. Colors represent kinases with exercise-regulated activity as shown in (A). Additional kinases (grey) and substrates (white) contain regulated phosphorylation sites.