Abstract
Purpose of review
Natural killer (NK) cells are innate lymphoid cells specialized to eliminate malignant cells via direct cytotoxicity and immunoregulatory cytokine production. As such, NK cells are ideal as cellular therapy for cancer patients, and a number of studies have provided proof-of-principle that adoptively transferred NK cells can induce remissions in patients with leukemia. A clear understanding of the mechanisms underlying NK cell anti-tumor responses, including target cell recognition, activation status, and negative regulatory signals will improve NK cellular therapy for cancer patients.
Recent findings
Clinical studies have demonstrated the safety and preliminary efficacy of NK cell adoptive transfer, especially in hematologic malignancies. A variety of NK cell sources, isolation techniques, activation approaches, and ex vivo expansion strategies are under investigation. New approaches have been developed and are being tested to optimize NK cell therapy, including ways to better target NK cells to malignant cells, increase their functional competence, facilitate expansion in patients, and limit inhibitory signals or cells.
Summary
NK cells represent a promising cellular immunotherapy for the treatment of cancer. In addition to adoptive cellular therapy, adjunct treatments that optimize NK cell targeting and function will enhance their potency and broaden their potential use to many cancer types.
Keywords: Natural Killer Cells, Immunotherapy, Cancer
Introduction
For over a century it has been understood that the immune system is involved in controlling tumor growth [1]. Cancer immunotherapy strategies seek to harness the immune system’s implicit ability to recognize and eliminate malignant cells, mediated by T cells, natural killer (NK) cells, NK-T cells, B cells, dendritic cells, and macrophages [2]. As the original immune-based cellular therapy, allogeneic hematopoietic cell transplantation (HCT) has provided long-term, disease-free survival to patients with hematologic cancers [3]. This procedure suppresses a patient’s immune system to allow engraftment of the allogeneic donor’s immune system, which in turn eliminates “foreign” cancer cells, commonly referred to as a graft versus leukemia effect (GVL). The drawback of this treatment approach is the recognition of normal patient cells as “foreign,” thereby causing graft versus host disease (GVHD) – a major life-threatening complication. Thus, one rational approach to improve allogeneic HCT is to isolate specific anti-tumor immune cells, primarily T and NK cells, and utilize them for cell-based therapy. Indeed, the success of chimeric antigen receptor (CAR) modified T cells for the treatment of B cell malignancies has demonstrated the promise of this reductionist cellular immunotherapy approach. Similarly, NK cells have been isolated from allogeneic donors, and utilized to induce remissions, primarily in patients with acute myeloid leukemia (AML).
NK cells are innate lymphoid cells that circulate through most tissues, and are specialized to eliminate virus-infected and malignantly transformed target cells [4,5]. NK cells contribute to cancer immunoediting [6], and are frequently deficient or dysfunctional in cancer patients [7–9], suggesting that NK cells represent a significant immunoevasion requirement for cancer genesis and progression. Further, a large epidemiologic study demonstrated that low NK cell function predicted for an increased risk of developing cancer [10]. In the setting of allogeneic HCT, HLA-haploidentical NK cells can recognize AML blasts, which predicts for improved outcomes in high risk AML [11–14]. Adoptive NK cell therapy studies utilizing the HLA-disparity between the donor NK cells and patient AML to target NK cells to blasts show promise [15,16]. Further, immunogenetic studies of killer-cell immunoglobulin-like receptors (KIR) in patients who have undergone HCT have correlated certain KIR haplotypes or activating receptor expression with disease relapse [17–19]. A number of parameters are currently being investigated to improve NK cell adoptive immunotherapy, including the donor cell source, the use of large-scale ex vivo expansion, use of off-the-shelf cell lines, and NK cell differentiation from progenitors [20–23]. Moreover, strategies are now being tested to optimize NK cell responses, including targeting NK cells more effectively to the tumor, enhancing NK cell anti-tumor functional status, and removing inhibitory signals or cells [23] . The focus of this review is to summarize recent advancements in the adoptive NK cellular therapy of cancer, and highlight promising NK cell immunotherapy combination strategies.
What is an NK Cell?
NK cells are innate lymphoid cells that can recognize and eliminate malignant cells. NK cell functions that are responsible for tumor surveillance and clearance include cytokine/chemokine secretion and cytotoxicity [4,5,24]. These cytokines (e.g. IFN-γ and TNF) and chemokines (e.g., MIP-1α) are important for shaping the immune response to the tumors and for recruiting additional effector cells to the site of malignancy [4]. NK cells also release perforin and granzymes into specialized cytotoxic synapses to induce target cell death. Following activation, NK cells may also express the death receptor ligands TRAIL and FasL, which bind to their receptors on target cells to trigger apoptosis. Human NK cells are phenotypically identified as CD56+ cells lacking T (CD3, TCR) and B (CD19) cell lineage markers, constitute approximately 10% of human blood lymphocytes, and consist of two developmentally-related but functionally-distinct subsets of human NK cells, CD56dim and CD56bright NK cells [25].
In order to maintain proper tolerance to healthy tissues and effectively eliminate diseased cells, NK cells utilize germ-line encoded activating and inhibitory receptors [26]. During development, NK cells must express at least one inhibitory receptor specific for self MHC class I (MHC-I) to attain functional competence (e.g. licensing) [27]. In a mature, licensed NK cell, the balance of signals received through these receptors determines the fate of the engaged cell, where more activating receptor signaling results in target killing and cytokine production. NK cells recognize diseased cells that have lost inhibitory receptor ligand expression (“missing self”) and upregulated activating receptor ligands (“abnormal or induced self”). KIR and C-type lectin inhibitory receptors (CD94/NKG2A) recognize MHC-I and MHC-I like molecules that are expressed on most normal healthy tissues, and provide the negative signals that prevent NK cell autoimmunity. Activating receptors expressed by NK cells, such as NKG2D, NKp46, and natural cytotoxicity receptors, recognize ligands that are upregulated on stressed or malignant cells [26]. NK cell receptors are variably and stochastically expressed on individual NK cells, resulting in thousands of NK cell specificities [28]. NK cell responses are not static; activated NK cells upregulate both inhibitory molecules (e.g., LAG-3, TIM-3, PD-1) and co-activating receptors (e.g., CD137). While induced inhibitory receptors are important in resolving a normal immune response and protecting healthy tissues, they also represent a means by which malignant cells evade NK cell responses [29–33]. Although immune checkpoints are well-defined in T cell anti-tumor responses, relevant activation-induced NK cell inhibitory checkpoints remain under investigation. In addition, recent studies revealed that NK cells can exhibit memory of prior activation [34] and NK cell memory is an area of active investigation [35]. Thus, NK cells are specialized effectors poised to respond to malignant cells, but in many diseases require modulation of trigger/recognition, functional capacity, or negative regulators for an optimal response.
Adoptive NK cell Immunotherapy
Since donor NK cell alloreactivity against leukemia correlated with improved clinical outcomes in AML patients with HLA-haploidentical HCT [11–14], most studies of NK cell adoptive immunotherapy have been performed in this disease. The recognition of an AML blast was linked to functionally-competent NK cells triggered via missing-self or induced/abnormal-self signaling [17–19]. In the first clinical trial investigating NK cell adoptive transfer outside of HCT, Miller et. al. enriched NK cells by depleting T cells (~40% NK cells in the final product), activated them overnight with IL-2, and administered them to lymphodepleted (fludarabine/cyclophosphamide) AML patients [15]. Key parameters of this and other reported studies of NK cellular therapy are summarized in Table 1. Overall, 5 of 19 (26%) of AML patients achieved a complete remission (CR) on this trial. Curti et. al., administered purified CD56+CD3− NK cells from HLA-haploidentical donors selected for a KIR-KIR ligand mismatch using a similar treatment protocol. Of the 5 patients with active AML, 1 patient (20%) obtained a CR [14]. More recently, Bachanova et. al. reported a series of patients treated with modifications of the Miller et al. platform, and demonstrated that the provision of IL-2-diptheriatoxin to deplete regulatory T cells (Tegs) enhanced NK cell expansion and increased the frequency of CR (50%). General conclusions from these studies included 1) lymphodepletion is important for donor NK cell expansion and resulted in increased host IL-15 production, 2) in vivo expansion of donor NK cells correlated with AML CR frequencies, 3) allogeneic NK cells did not cause GVHD, 4) patient Tregs limit NK cell expansion and anti-leukemia activity in vivo. Multiple groups are examining alternative sources of NK cells, such as ex vivo expansion, differentiation from progenitors, and even immortalized NK cell lines [21,36–39]. While these studies provide proof-of-principle that allogeneic NK cell adoptive transfer may induce remissions in leukemia patients, it is clear that new, complementary approaches are needed to achieve lasting responses in patients (Table 2).
Table 1.
Summary of recent NK cell adoptive therapy clinical trials. Flu, intravenous fludarabine; Cy,intravenous cyclophosphamide; IL2DT, IL-2-diphtheria fusion protein; A, Adult; P, Pediatric; CD3−, CD3 depletion; CD56+, CD56, positive selection; CD19−, CD19 depletion; NR, not reported. CR rates include those patients with active disease at the time of therapy.
| Study | Conditioning | Post-Infusion Therapy IL-2 (×106 IU/m2) |
KIR-KIR ligand Mismatch |
Purification | NK Dose (× 106/kg) |
Donor Chimerism |
Donor NK cell number (per μl) |
Peak Expansion (Days) |
CR | GVHD | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Rubnitz et
al., 2010 |
Flu-Cy | 1.0 | Yes | CD3-CD56+ | 29 | 7 % (NK) | 5.8 | 14 | NA | No | [13] |
|
Curti et al.,
2011 |
Flu-Cy | 1.0 | Yes | CD3-CD56+ | 2.5 | NR | NR | 7-10 | 1/5 | No | [14] |
|
Miller et al.,
2005 |
Flu-Cy | 1.75-10 | No | CD3- | 9 | 25 % (PBMC) | NR | 14 | 5/19 | No | [15] |
|
Bachanova
et al., 2014 |
Flu-Cy | 9.0 | 7 | [16] | |||||||
| Cohort 1 | 17% | CD3- | 9.6 | NR | NR | 5/19 | No | ||||
| Cohort 2 | 50% | CD3-CD56+ | 3.4 | NR | NR | No | |||||
| Cohort 3 | +IL2DT | 56% | CD3-CD19− | 26 | 49 % (PBMC) | 190 | 8/15 | No |
Table 2.
Recently published studies aimed at enhancing NK cell tumor responses by increasing NK cell activity, increasing NK cell specificity, and/or decreasing NK cell suppression.
| Preclinical studies | |||||
|---|---|---|---|---|---|
| Disease | Cell Product/ Agent |
Description | Outcomes | NK Cell Strategy |
Reference |
|
Hematologic
malignancy |
NK cells | Preactivation of cord blood NK cells by cytokines |
Cord blood NK cells pre-activated with cytokine combinations (IL-15 + IL-2 or IL-18) are more active than those pre-activated with IL-2 alone |
↑Activity Preclinical |
[36] |
| MDS | Bispecific Killer Engager (BiKE) |
CD16xCD33 BiKE to target NK cells to CD33+ MDS |
CD16xCD33 BiKE increases activity of intact MDS patient NK cells, ex vivo |
↑Specificity ↓ Suppression Preclinical |
[40] |
|
Hodgkin
Lymphoma |
AFM13 Bispecific Ab construct (TandAb) |
CD30/CD16A TandAb to recruit NK cells to lyse CD30+ tumors |
Tetravalent bispecific CD30/CD16A is optimized for NK cell ADCC and engagement the TandAb increased NK cell cytotoxicity in a CD30/CD16A dependent manner |
↑Specificity Preclinical |
[41] |
|
CLL &
WM Lymphoma |
Ublituximab Fc- Optimized mAb |
ADCC optimized α-CD20 compared to standard α- CD20 |
ADCC optimized anti CD20 antibody Ublituximab enhanced degranulation and cytotoxicity of NK cells in response to opsonized targets compared to rituximab treated controls |
↑Specificity Preclinical |
[42, 43] |
|
Prostate
Cancer |
DAP12-based CAR NK cells |
YTS NK Cell line was transduced to express an α-PSCA-DAP12 CAR |
DAP12 CAR-YTS NK cells were more cytotoxic than CD3ζ CAR YTS NK cells. Primary NK cells engineered with the DAP12-CAR recognized and killed PSCA+ tumor cells in vitro |
↑Specificity Preclinical |
[44] |
|
Multiple
Myeloma |
CD3/CD28 CAR NK Cells |
NK cell lines engineered to express CS1-specific CAR |
Two NK cell lines, NK-92 and NKL, killed tumor targets in a CS1 dependent manner |
↑Specificity Preclinical |
[45] |
| Carcinoma | Anti-41BB, cetuximab |
α-41BB enhances NK cell function against cetuximab (α-EGFR) coated tumor targets |
Cetuximab triggers ADCC by NK cells which is enhanced by ligation of the 41BB agonistic mAb |
↑Activity (α-41 BB) ↑Specificity (cetuximab) Preclinical |
[46] |
| Melanoma | α-TIM-3 mAb | Hyporesponsive NK cells from patients treated with α-TIM-3 mAb |
TIM-3 blockade restores anti-tumor responses of melanoma patient-derived NK cells, in vitro |
↓ Suppression Preclinical |
[47] |
| Clinical studies | |||||
| Disease |
Cell Product/
Agent |
Description | Outcomes |
NK Cell
Strategy |
Reference |
| AML | IL-2 diphtheria toxin fusion, NK cells, IL-2 |
IL-2DPT pretreatment + IL-2 preactivated haplo- identical NK cells |
IL-2DP effectively depleted Tregs and improved engraftment of IL-2 preactivated, haploidentical NK cells in AML patients |
↑Activity ↓ Suppression Clinical |
[16] |
|
Melanoma
Renal Cell cancer |
Recombinant human IL-15 |
Determine the safety of intravenous bolus IL-15 administration |
IL-15 can be administered safely, however alternative strategies will likely increase efficacy and reduce toxicity |
↑Activity Clinical |
[48] |
|
Rel/Ref
Multiple Myeloma |
Anti-KIR antibody IPH2101 and Lenalidomide |
α-KIR mAb plus lenalidomide to treat patients |
IPH2101 plus lenalidomide is safe, tolerable and evidence suggests may lengthen progression free survival. |
↑Activity ↓ Suppression Clinical |
[49] |
There remain a large number of open questions in the field, including the optimal setting in which to administer adoptive NK cell therapy. Although NK cell adoptive immunotherapy has the benefit of no GVHD, the “window of opportunity” for NK cells to clear leukemia is a few weeks, since host T cells eliminate allogeneic donor NK cells as they recover from Flu/Cy. In contrast, adding NK cell infusions to HCT procedures may allow for persistence in the host, enhance GVL and reduce GVHD, but does not address the key adverse events associated with the HCT per se. Thus, the most recent research efforts have investigated multiple approaches to enhance NK cell anti-tumor responses, which may have implications in NK cell adoptive immunotherapy in either setting, and may also be used to stimulate endogenous patient NK cell responses. The remainder of the review focuses on progress in the three major strategies to enhance NK cell-mediated tumor clearance.
Providing a trigger: enhancing NK cell tumor recognition
While initial studies utilized KIR ligand mismatch to facilitate donor NK cell recognition of recipient leukemias, advances in our understanding of NK cell triggering via CD16 by monoclonal antibody therapies has led to the development of novel single-chain variable fragments (scFv) fusion proteins designed to enhance targeting the NK cell to the tumor. Bispecific and trispecific killer cell engagers (BiKE and TriKE) that cross-link CD16 expressed on NK cells and 1-2 tumor antigens on target cells have been reported in pre-clinical studies [50]. The CD16xCD33 BiKE triggered NK cells via CD16 to kill and produce cytokines in response to CD33-expressing cell lines and primary leukemia samples in vitro [50]. Gleason et al. have recently tested the effectiveness of the CD16xCD33 BiKE to target NK cells to myelodysplastic syndrome (MDS) patient samples (CD33+ MDS targets) [40]. Moreover, it was demonstrated that the CD16xCD33 BiKE triggers patient NK cells to lyse CD33+ MDS blasts and immunosuppressive CD33+ myeloid derived suppressor cells (CD33+ MDSCs) in intact patient samples. Thus, CD33-scFv BiKEs could be utilized to limit endogenous myeloid regulators of NK cells, thereby enhancing allogeneic, and potentially autologous, NK cell responses. Rothe et al. reported a bispecific NK cell-specific targeting agent, AFM13, in a phase 1 study for treating rel/ref Hodgkin lymphoma [41,51]. AFM13 is a tetravalent chimeric antibody construct (TandAb) that contains two binding domains for CD16A, the NK cell specific isoform of the CD16 receptor, and CD30, which is expressed on hematologic malignancies, including Hodgkin lymphoma. In this study, patients were treated with escalating doses of AFM13 (0.01 to 7.0 mg/kg, three patients per dose) administered once a week for 4 weeks. AFM13 was well tolerated, and 3 of 26 patients experienced a partial response. Furthermore, the authors demonstrated enhanced activation marker expression on NK cells after AFM13 infusion providing a proof-of-principle that NK cells can be targeted and enhanced by AFM13 in vivo. By specifically using the CD16A scFv the large sink of CD16+ non-NK cells is eliminated (e.g. CD16B+ neutrophils), potentially improving the potency of this class of agents. Finally, a number of clinical monoclonal antibodies partially rely on ADCC mediated tumor clearance, including trastuzumab, rituximab, and cetuximab [52–55]. Efforts are being made to optimize monoclonal antibodies to enhance ADCC and antibody-dependent cytokine release (ADCR) by NK cells [42,56]. Because these responses are dependent on IgG Fc interaction with Fc receptors, de Romeuf et al. generated a chimeric monoclonal antibody which promoted optimal FcγRIIIA (CD16A) binding and signaling [56]. In two separate studies, Le Garff-Tavernier et. al., compared ADCC of CLL or lymphoplasmacytic lymphoma opsonized with the Fc-optimized anti-CD20 monoclonal antibody, ublituximab, or with the first-generation anti-CD20 antibody, rituximab [43,57]. In both studies, NK cell engagement with the ublituximab resulted in increased degranulation and target killing compared to rituximab [43,57]. All of these agents rely on CD16-triggered NK cell functions, however, a number of additional activating receptors (e.g. NKG2D) expressed by NK cells could be targeted in a similar fashion, thereby producing potent activating signals to enhance NK cell effector functions. Apart from these antibody-type targeting agents, there is interest in using CAR-modified NK cells for enhancing tumor specificity [44,45,58,59]. Haploidentical NK cells could be enriched from donor PBMCs, transduced to express tumor-specific CAR with NK cell activating receptor signaling domains (i.e., DAP10 and DAP 12) [45]. Because aplasia of normal hematopoietic stem cells and progenitors induced by long-lasting CAR T cells is currently one of the major hurdles in successfully using this technology for treating cancers like AML [60,61], the shorter persistence of allogeneic CAR NK cells may provide a viable alternative to CAR T cells.
Press the gas pedal: function-enabling NK cells
NK cell functional capacity can be enhanced in a variety of ways to improve NK cell mediated tumor clearance. Constitutive and induced expression of cytokine receptors by NK cells provides opportunities to use cytokines for immunomodulation [62]. Classically, IL-2 has been used to activate and support NK cells in vivo for patients receiving NK cell adoptive therapy. One major drawback is the exquisite sensitivity of Tregs to IL-2 via their high affinity IL-2Rαβγ, which may expand and limit NK cell responses [62]. IL-15 is the critical cytokine for NK cell homeostasis and function, and has been a long-standing attractive alternative to IL-2 to augment NK cell and CD8 T cell number and function [48,63,64]. Recently, the results of a first-in-human phase 1 clinical trial of rhIL-15 administered to advanced cancer patients demonstrated safety with clear NK and T cell immunomodulation [65]. This study provides the first evidence that IL-15-based agents are feasible in the clinic with favorable adverse event profiles at biologically active doses. Alternative forms of IL-15 based therapy have also been developed and are now in clinical trials. ALT-803 is an IL-15 super agonist mutein complexed with a fusion of IL-15Rα sushi domains to an IgG1 Fc domain, resulting in increased stability and in vivo half-life of this protein complex [66]. ALT-803 is currently being evaluated in a number of clinical trials in cancer patients, and more recently in combination with therapeutic mAbs. IL-12, IL-18, and IL-21 are also being explored as NK cell activating cytokine adjuvants [62]. Beyond the scope of this review, several groups are utilizing cytokines and artificial stimulator cells to expand and activate NK cells prior to adoptive transfer (Table 3) [62,67].
Table 3.
Ongoing clinical trials utilizing NK cells and NK cell functional modulation to treat cancer.
| Clinical Diagnosis | ClinicalTrials.gov Identifier |
Trial Phase | Cell Product / Agent | Institute(s) Involved |
|---|---|---|---|---|
| AML and MDS | NCT01898793 | Phase I | Cytokine Induced Memory Like NK Cells |
Washington University School of Medicine |
| AML | NCT01370213 | Phase II | IL-2 activated NK cells prior to αβ-depleted haploidentical hematopoietic cell transplant |
University of Minnesota, Washington University School of Medicine, Emory University, and Ohio State University |
| AML and MDS | NCT01385423 | Phase I | NK cells followed by rh-IL15 | University of Minnesota |
|
Pediatric patients
with leukemia or solid tumors |
NCT01287104 | Phase I | Ex vivo expanded NK cells early after hematopoietic cell transplant |
National Cancer Institutes (NCI) |
| Leukemia | NCT01823198 | Phase I/II | NK cells with HCT for high risk Myeloid malignancy |
MD Anderson Cancer Center |
|
Hematologic
malignancies |
NCT00789776 | Phase I/II | NK cell infusion after hematopoietic cell transplant |
Fred Hutchinson Cancer Center, Children’s Hospital of Milwaukee and Medical College of Wisconsin |
| Leukemia |
NCT01619761 NCT02280525 |
Phase I | Expanded cord blood NK cells | MD Anderson |
|
Hematologic
malignancies |
NCT01853358 | Phase I | IL-2 activated NK cells | Institut Paoli-Calmettes, France |
|
Hematologic
malignancies |
NCT01904136 | Phase I/II | IL-2 activated NK cells with hematopoietic cell transplant |
MD Anderson Cancer Center |
| AML | NCT01787474 | Phase I | IL-21 expanded NK cells | MD Anderson Cancer Center |
| Multiple Myeloma | NCT01040026 | Phase I/II | Ex vivo expanded allogeneic NK cells with autologous hematopoietic cell transplant |
University Hospital, Basel, Switzerland |
| Lymphoma | NCT01956695 | Phase I | Efficacy of Lenalidomide with rituximab in rel/ref primary CNS lymphoma |
Institute Curie, France |
| Leukemia | NCT01807611 | Phase II | KIR mismatched haploidentical NK cell transplant |
St. Jude Children’s Research Hospital |
| AML | NCT00900809 | Phase I | NK cell line (NK-92, Neukoplast) |
UPMC Cancer Center |
| Lymphoma | NCT01729104 | Phase I/II | Carfilzomib plus lenalidomide and rituximab in the treatment of rel/ref MCL |
MD Anderson Cancer Center |
| ALL | NCT02185781 | Phase I | Expanded autologous NK cells | Policlinico Umberto I di Roma |
| Leukemia | NCT02420938 | Phase II | Urelumab with rituximab for rel/ref or high-risk CLL |
MD Anderson Cancer Center |
| AML | NCT01520558 | Phase I/II | CNDO-109-AANK for AML in First Complete Remission |
Moffitt Cancer Center, University of Minnesota, Washington University School of Medicine, Medical University of South Carolina |
|
Hematologic
malignancies |
NCT01885897 | Phase I/II | Safety and efficacy of ALT-803 for relapse of malignancy after Allogeneic SCT |
University of Minnesota |
| iNHL | NCT02384954 | Phase I/II | Safety and efficacy of ALT-803 for rel/ref iNHL plus rituximab |
Washington University School of Medicine |
| Multiple Myeloma | NCT02099539 | Phase I/II | Safety and efficacy of ALT-803 for rel/ref multiple myeloma |
University of Minnesota, Washington University School of Medicine, Roswell Park Cancer Center, Thomas Jefferson University |
|
Breast and Gastric
Cancer |
NCT02030561 | Phase II | NK cells + Trastuzumab | National University Hospital, Singapore |
Recent advances in NK cell biology have identified that NK cells exhibit a memory of prior activation [34], which includes enhanced responses to leukemia. For example, brief IL-12, IL-15, and IL-18 combined pre-activation of human NK cells resulted in differentiation of memory-like NK cells [68,69], with enhanced IFN-γ secretion and cytotoxicity upon restimulation with cytokines or tumor targets [35,68]. This approach is now being tested in a first-in-human study of cytokine induced memory-like NK cells in patients with relapsed or refractory AML (NCT01898793). Phase 1 testing of haploidentical NK cells primed ex vivo with CTV-1 lysate has been completed, and preliminary reports show that this approach is safe (NCT01520558) [70]. Lenalidomide is an immunomodulatory agent that has been demonstrated to enhance ADCC by NK cells [46,71]. Multiple trials are utilizing lenalidomide in combination with anti-tumor monoclonal antibodies, in order to enhance NK cell mediated ADCC and to improve clinical outcomes for patients (Table 2). In addition to targeting NK cells to tumors, monoclonal antibodies can also signal through costimulatory receptors to improve NK cell function. Using anti-41BB agonistic antibodies is one such strategy which is currently being tested in the clinic (Table 2) [72].
Take your foot off the brake: blocking NK cell inhibition
Similar to all other immune cells, NK cells have endogenous checks on their activity. To fully optimize NK cell anti-tumor responses in vivo, approaches are required to limit NK cell suppression, either from inhibitory molecules expressed by the NK cell (i.e. KIR) [73], or by suppressor cell types (i.e. regulatory T cells, myeloid derived suppressor cells) [74,75] (Figure 1). As previously mentioned, Tregs represent one obstacle to proper NK cell expansion after adoptive therapy plus IL-2 [47]. In order to limit Tregs during IL-2 administration, Bachanova et al. utilized an IL-2 diphtheria toxin fusion protein (IL2DT) to selectively deplete recipient Tregs [16]. In addition to extrinsic NK cell inhibition by regulatory cells, inhibitory receptors expressed by NK cells can also suppress their activities, such as the constitutively expressed KIR or NKG2A, as well as the induced co-inhibitory receptors (i.e. TIM-3, PD-1) [26,76]. IPH2101 is a mAb blocking common KIRs that bind to HLA-C alleles with the aim of disrupting the inhibitory HLA-KIR signal and enhancing NK cell function [77,78,49]. Initial clinical studies using IPH2101 did not demonstrate any major responses while using this as a single agent [77,78]. In a recent study, IPH2101 combined safely with lenalidomide in the absence of steroids with objective responses observed in patients with rel/ref multiple myeloma [49], providing support for the concept that combined immunotherapy strategies are required to unleash the most potent NK cell response.
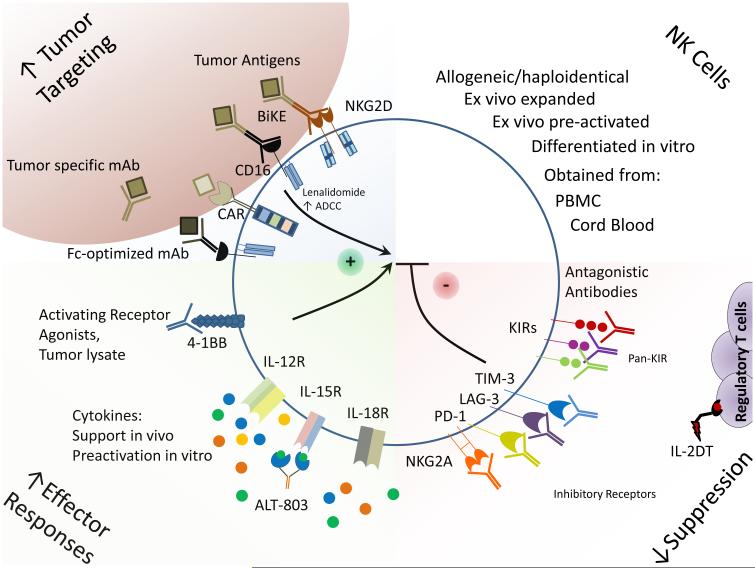
Figure 1. Combinatorial strategies to improve NK cell adoptive immunotherapy.
NK cell adoptive therapy can be improved by combination approaches aimed at enhancing NK cell tumor specificity, enhancing NK cell effector capacity, and by reducing NK cell inhibition. Tumor-specificity can be increased by utilizing bispecific killer engagers (BiKE) or ADCC-optimized, tumor-specific monoclonal antibodies. NK cell effector capacity can be improved by in vitro preactivation with cytokines as well as in vivo with cytokine support, either with recombinant cytokines or with cytokine receptor super-agonists (e.g. ALT-803). Furthermore, activating receptor agonists can also be employed in vivo to improve NK cell activation and effector responses. Antibody checkpoint blockade therapy specific for inhibitory receptors expressed by NK cells will limit cell intrinsic immunosuppression. Finally, efforts to deplete regulatory T cells during NK cell adoptive therapy, e.g., using IL-2 diphtheria toxin fusion protein (IL2DT), are promising and currently under investigation.
Conclusions
The great promise of NK cell anti-tumor immune effects continues to expand as basic aspects of their biology are unraveled and translated to the clinic. Adoptively transferred NK cells mediate anti-leukemia responses and can result in remissions, but many open questions remain regarding optimal purification, ex vivo manipulations, and in vivo support tactics. New immunotherapy approaches that combine allogeneic NK cell therapy with strategies to 1) improve targeting/triggering, 2) augment anti-tumor responses, and 3) limit inhibition will be the key to enhance the efficacy of NK cell adoptive therapy to a wider variety of malignancies.
Key Points.
NK cell adoptive immunotherapy may induce complete remissions in patients with acute myeloid leukemia
NK cell recognition and targeting may be enhanced using therapeutic mAbs, bi- and tri-specific agents, and chimeric antigen receptors
NK cells are primed for anti-tumor responses by cytokines, activating receptor mAbs, and immunomodulatory agents
Checkpoints on NK cell anti-tumor responses include inhibitory receptor signals and suppressive cells that may be targeted to enhance NK cell therapy
Acknowledgments
We would like to thank members of the Fehniger laboratory for insightful discussion.
Financial support and sponsorship
This work was supported by NIH F32 CA200253 (MMB-E), American Society of Hematology Foundation Scholar Award (RR), Washington University School of Medicine Siteman Cancer Center (TAF), the Cancer Frontier Fund (TAF), the Gabrielle’s Angel Foundation for Cancer Research (TAF), and NIH R01 AI102924 (TAF).
Footnotes
Conflicts of interest
TAF participated in a Coronado Bioscience Scientific Advisory Board in 2015. The remaining authors have no potential conflicts of interest.
References
- 1.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. Cancer Immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 3.Copelan EA. Hematopoietic Stem-Cell Transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 8.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- 9.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemic cells in NCRdull phenotype induction. Blood. 2006;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Matsuyama S, Miyake S, Suga K, Yu H, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 12.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr. Opin. Immunol. 2009;21:525–530. doi: 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui C-H, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high-risk acute myeloid leukemia patients. Blood. 2011 doi: 10.1182/blood-2011-01-329508. doi:10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 15.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney S a, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 16.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **. This study provides in vivo evidence in patients that Tregs compete with and limit the success of allogeneic NK cell adoptive immunotherpay.
- 17.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, Marsh SGE, Guethlein L a, Parham P, Miller JS, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–32. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooley S, Weisdorf DJ, Guethlein L a, Klein JP, Wang T, Le CT, Marsh SGE, Geraghty D, Spellman S, Haagenson MD, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–9. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venstrom JM, Pittari G, Gooley T a, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–16. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy WJ, Parham P, Miller JS. NK cells--from bench to clinic. Biol Blood Marrow Transpl. 2012;18:S2–7. doi: 10.1016/j.bbmt.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geller MA, Miller JS. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3:1445–59. doi: 10.2217/imt.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JS. Therapeutic applications: natural killer cells in the clinic. Hematology Am. Soc. Hematol. Educ. Program. 2013;2013:247–53. doi: 10.1182/asheducation-2013.1.247. [DOI] [PubMed] [Google Scholar]
- 23.Ljunggren H-G, Malmberg K-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 24.Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9:473–5. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- 25.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–56. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanier LL. NK cell receptors. Annu. Rev. Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson HA, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *. First application of mass cytometry to define NK cell diversity. This approach will become a standard immunomonitoring tool for NK cell based immunotherapy of cancer.
- 29.Nguyen S, Beziat V, Dhedin N, Kuentz M, Vernant JP, Debre P, Vieillard V. HLA-E upregulation on IFN-gamma-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2009;43:693–699. doi: 10.1038/bmt.2008.380. [DOI] [PubMed] [Google Scholar]
- 30.Gleason MK, Lenvik TR, Mccullar V, Felices M, Brien MSO, Sarah A, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-mortari A, et al. interferon gamma production in response to galectin-9 Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, Triebel F, Charron D, Aoudjit F, Al-Daccak R, et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J. Immunol. 2011;186:5173–5183. doi: 10.4049/jimmunol.1002050. [DOI] [PubMed] [Google Scholar]
- 32.Chamuleau MED, Souwer Y, Van Ham SM, Zevenbergen A, Westers TM, Berkhof J, Meijer CJLM, Van De Loosdrecht A a, Ossenkoppele GJ. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–5550. doi: 10.1158/0008-5472.CAN-04-1350. [DOI] [PubMed] [Google Scholar]
- 33.Farnault L, Sanchez C, Baier C, Le Treut T, Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: Mechanisms and therapeutic implications. Clin. Dev. Immunol. 2012:2012. doi: 10.1155/2012/421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–8. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berrien-Elliott MM, Wagner J a., Fehniger T a. Human Cytokine-Induced Memory-Like Natural Killer Cells. J. Innate Immun. 2015 doi: 10.1159/000382019. Apr 30:[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alnabhan R, Madrigal A, Saudemont A. Differential activation of cord blood and peripheral blood natural killer cells by cytokines. Cytotherapy. 2015;17:73–85. doi: 10.1016/j.jcyt.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Childs RW, Berg M. Bringing natural killer cells to the clinic: ex vivo manipulation. Hematology Am. Soc. Hematol. Educ. Program. 2013;2013:234–46. doi: 10.1182/asheducation-2013.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng X, Wang Y, Wei H, Sun R, Tian Z. LFA-1 and CD2 synergize for the Erk1/2 activation in the Natural Killer (NK) cell immunological synapse. J Biol Chem. 2009;284:21280–7. doi: 10.1074/jbc.M807053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Gleason MK, Ross J a., Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **. This study demonstrates that multi-specific proteins can enhance NK cell anti-tumor activity by targeting the NK cells to the tumor of interest in a specific fashion.
- 41.Reusch U, Burkhardt C, Fucek I, Le Gall F, Le Gall M, Hoffmann K, Knackmuss SHJ, Kiprijanov S, Little M, Zhukovsky E a. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Garff-Tavernier M, Herbi L, de Romeuf C, Nguyen-Khac F, Davi F, Grelier a, Boudjoghra M, Maloum K, Choquet S, Urbain R, et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia. 2014;28:230–3. doi: 10.1038/leu.2013.240. [DOI] [PubMed] [Google Scholar]
- 43.Le Garff-Tavernier M, Herbi L, de Romeuf C, Azar N, Roos-Weil D, Bonnemye P, Urbain R, Leblond V, Merle-Béral H, Vieillard V. The optimized anti-CD20 monoclonal antibody ublituximab bypasses natural killer phenotypic features in Waldenstrom macroglobulinemia. Haematologica. 2015;100:147–151. doi: 10.3324/haematol.2014.118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topfer K, Cartellieri M, Michen S, Wiedemuth R, Muller N, Lindemann D, Bachmann M, Fussel M, Schackert G, Temme a. DAP12-Based Activating Chimeric Antigen Receptor for NK Cell Tumor Immunotherapy. J. Immunol. 2015 doi: 10.4049/jimmunol.1400330. doi:10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- *. Chimeric antigen receptor approaches in NK cells may be tailored to NK cell signaling.
- 46.Ghosh N, Grunwald M, Fasan O, Bhutani M. Expanding role of lenalidomide in hematologic malignancies. Cancer Manag. Res. 2015;7:105–119. doi: 10.2147/CMAR.S81310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol. Immunother. 2010;59:1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 49.Benson DM, Cohen a. D, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, Efebera Y a., Andre P, Zerbib R, Caligiuri M a. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0304. doi:10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *. Clinical combination of NK cell functional enhancement (lenalidomide) in combination with inhibitory recetpor blockade (IPH2101)
- 50.Gleason MK, Verneris MR, Todhunter D a, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera D a, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer Ther. 2012;11:2674–84. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothe A, Sasse S, Topp MS, Eichenauer D a., Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, et al. A phase I study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015 doi: 10.1182/blood-2014-12-614636. COI 10.118:[Epub May 6 2015 ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *. Promising TandAb bi-specific agent that selectively triggers NK cells via CD16A.
- 52.Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–4489. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, López-Albaitero A, Gibson SP, Gooding WE, Ferrone S, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *. Highlights the promise of innate NK cell based therapy to augment antigen specific immunity, resulting in durable immune responses to cancer.
- 54.Veeramani S, Wang S-Y, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–9. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang K-Y, Schlom J. Antibody dependent cellular cytotoxicity (ADCC) activity of a novel anti-PD-L1 antibody, avelumab (MSB0010718C), on human tumor cells. Cancer Immunol. Res. 2015 doi: 10.1158/2326-6066.CIR-15-0059. doi:10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Romeuf C, Dutertre CA, Le Garff-Tavernier M, Fournier N, Gaucher C, Glacet A, Jorieux S, Bihoreau N, Behrens CK, Béliard R, et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcγRIIIA/CD16. Br. J. Haematol. 2008;140:635–643. doi: 10.1111/j.1365-2141.2007.06974.x. [DOI] [PubMed] [Google Scholar]
- 57.Le Garff-Tavernier M, Herbi L, de Romeuf C, Nguyen-Khac F, Davi F, Grelier a, Boudjoghra M, Maloum K, Choquet S, Urbain R, et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia. 2014;28:230–3. doi: 10.1038/leu.2013.240. [DOI] [PubMed] [Google Scholar]
- *. Excellent example of of how ADCC-optimized therapeutic mAbs effectively enhance NK cell anti-tumor responses.
- 58.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–27. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 2015;6:1–7. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–54. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJD, Scholler J, Song D, Porter DL, Carroll M, et al. CD33 Specific Chimeric Antigen Receptor T Cells Exhibit Potent Preclinical Activity against Human Acute Myeloid Leukemia. Leukemia. 2015 doi: 10.1038/leu.2015.52. doi:10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014 doi: 10.1155/2014/205796. doi:10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 64.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–83. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 65.Conlon KC, Lugli E, Welles HC, Rosenberg S a, Fojo AT, Morris JC, Fleisher T a, Dubois SP, Perera LP, Stewart DM, et al. Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production During First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients With Cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **. First-in-human study of rhIL-15 showing the feasability, safety, and in vivo NK cell and T cell modulation. Proof-of-principle that IL-15 based therapy will replace rhIL-2 to suport NK cell number and funciton in vivo.
- 66.Xu W, Jones M, Liu B, Zhu X, Johnson CB, Edwards AC, Kong L, Jeng EK, Han K, Marcus WD, et al. Efficacy and Mechanism-of-Action of a Novel Superagonist Interleukin-15: Interleukin-15 Receptor αSu/Fc Fusion Complex in Syngeneic Murine Models of Multiple Myeloma. Cancer Res. 2013;73:3075–86. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **. Novel, promosing IL-15-based agent with transpresentation and prolonged in vivo pharmacokinetics.
- 67.Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling Natural Killer Cell Responses: Integration of Signals for Activation and Inhibition. Annu. Rev. Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 Induces CD25 and a Functional High-Affinity IL-2 Receptor on Human Cytokine-Induced Memory-like Natural Killer Cells. Biol Blood Marrow Transpl. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *. Along with [68] demonstates the immunotherapy translatioanl potential of memroy-like NK cells.
- 70.Fehniger TA, Stuart RK, Cooley SA, Miller JS, Curtsinger J, Hillman TM, Silver N, Szarek M, Lowdell MW, Gorelik L, et al. Preliminary Results of a Phase 1/2 Clinical Trial of Cndo-109-Activated Allogeneic Natural Killer Cells in High Risk Acute Myelogenous Leukemia Patients in First Complete Remission. Blood. 2014;124:2320. [Google Scholar]
- 71.Epling-Burnette PK, Bai F, Painter JS, Djeu JY, List AF. Lenalidomide effects on NK function in patients with MDS. Blood. 2005;106:#3437. [Google Scholar]
- 72.Kohrt HE, Colevas a. D, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R, et al. Targeting CD137 enhances the efficacy of cetuximab. J. Clin. Invest. 2014;124:2668–2682. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- *. This study demonstrates that combination approaches enhance NK cell activity and anti-tumor responses.
- 73.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 74.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J. Exp. Med. 2013 doi: 10.1084/jem.20122571. doi:10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Da Silva IP, Gallois a., Jimenez-Baranda S, Khan S, Anderson a. C, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-Cell Exhaustion in Advanced Melanoma by Tim-3 Blockade. Cancer Immunol. Res. 2014;2:410–22. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vey N, Bourhis J-H, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, et al. A phase I trial of the anti-inhibitory KIR monoclonal antibody IPH2101 for acute myeloid leukemia (AML) in complete remission. Blood. 2012 doi: 10.1182/blood-2012-06-437558. doi:10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 78.Benson DM, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, Bakan C, Andre P, Efebera Y, Tiollier J, et al. Aphase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **. This study demonstrates that combination approches to improve NK cell therapy hold clinical promise.