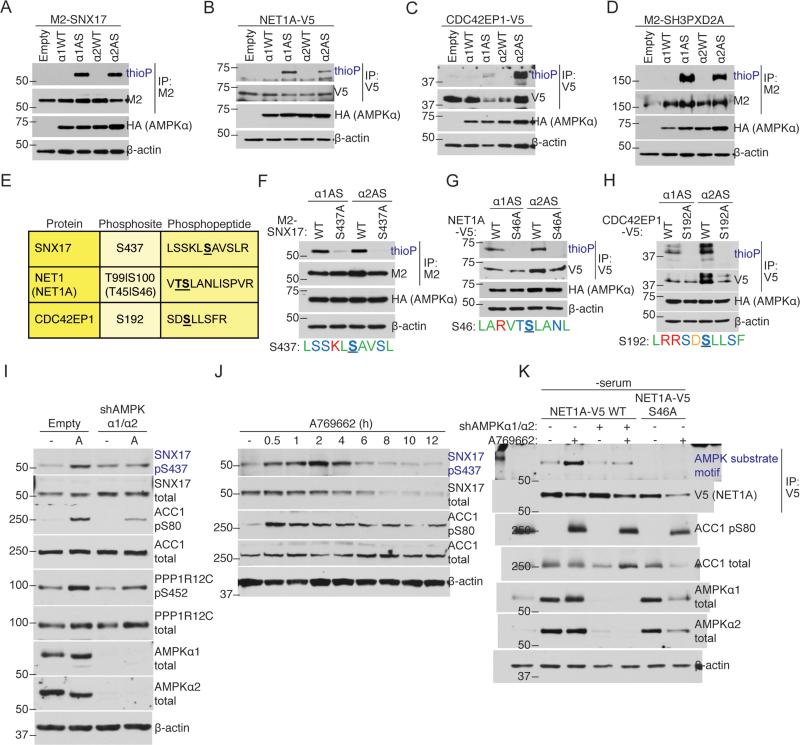
Figure 3. AS-AMPK directly phosphorylates several high confidence substrates, including SNX17 and NET1A.
(A-D) AS-AMPK thiophosphorylates SNX17, NET1A, CDC42EP1, and SH3PXD2A. Tagged proteins were over-expressed in empty vector or AMPKα-expressing (HA-tagged WT or AS-AMPKα1 or α2) U2OS cell lines, immunoprecipitated, and analyzed by western blot for the presence of thiophosphorylation. AMPK was activated in all conditions with 15 minutes of 50 mM 2DG. Representative of 3, 1, 1, and 2 independent experiments, respectively.
(E) Mass spectrometry-predicted AMPK phosphorylation sites and corresponding phosphopeptides for SNX17, NET1A, and CDC42EP1. Labeled as in Figure 1E. S100 on NET1 (S46 on the short isoform NET1A) was used as the NET1/NET1A site as its surrounding motif resembled the AMPK motif better than that of T99.
(F-H) AS-AMPK thiophosphorylates SNX17, NET1A, and CDC42EP1 at the identified residues. Tagged WT and predicted phosphorylation site mutants of the indicated substrates were over-expressed in U2OS AS-AMPKα2 and α1 cell lines, immunoprecipitated, and analyzed as in Figures 3A-D. The phosphorylation motif for the predicted residue is shown. The phosphorylated residue is underlined and bold. Color-coding as in Figure 2B. Each panel representative of 2 independent experiments.
(I) AMPK phosphorylates S437 on SNX17 endogenously. AMPK was activated in U2OS cells stably expressing an shRNA against AMPKα1 and α2 or empty vector control. Phosphorylation of the known substrates ACC1 S80 and PPP1R12C S452 are shown as controls for AMPK activation. Note that there is still some degree of AMPK substrate phosphorylation in cells with stable knockdown of AMPKα1 and α2, probably due to residual AMPK expression in these cells. “-“, no drug (DMSO vehicle control); A, A769662, 300 μM for 30 minutes. Representative of 2 independent experiments.
(J) Specific activation of AMPK decreases SNX17 protein levels. AMPK was activated in U2OS cells with 300 μM of A769662 for the indicated amount of time. Representative of 2 independent experiments.
(K) Over-expressed NET1A is phosphorylated at S46 in response to endogenous AMPK activation. NET1A-V5 WT or S46A was expressed in a doxycycline-inducible manner in U2OS cell lines. NET1A-V5 WT was also expressed in U2OS cell lines with shRNA-knockdown of both AMPKα1 and α2. Cells were serum-starved overnight, which was important to decrease basal NET1A phosphorylation, and NET1A-V5 expression was induced by 2 hours of doxycycline exposure (see Supplemental Experimental Procedures). AMPK was activated with 300 μM A769662 for 30 minutes. Following NET1A-V5 immunoprecipitation, samples were immunoblotted with an AMPK substrate motif antibody. Representative of 3 independent experiments.