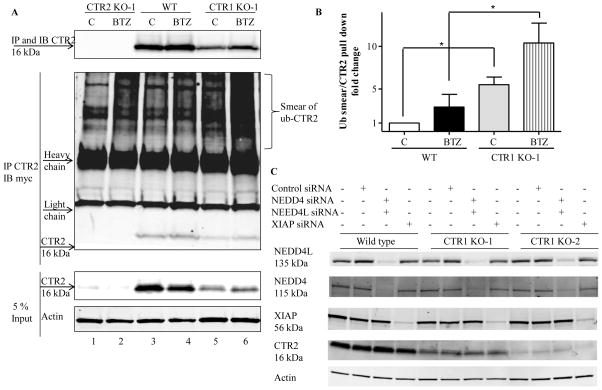
Figure 7.
CTR2 is subject to ubiquitination in CTR1 knockdown cells. Wild type HEK293T cells and the CTR2 KO-1 and CTR1 KO-1 clones were transfected with a vector expressing myc-tagged ubiquitin and 16 h later treated with 100 nM BTZ for 8 h. (A) Whole cell lysates were collected and equal amounts of total proteins were immunoprecipitated (IP) with anti-CTR2 and subjected to immunoblot (IB) analysis with anti-CTR2 (upper) and anti-myc antibody (middle). Five percent of the total protein was used as input (bottom). CTR2 KO-1 cells were used as a negative control for the anti-CTR2 antibody. The immunoblot shown is representative of 3 independent experiments. (B) Quantitation of ubiquitinated CTR2 smear to amount of CTR2 in the immunoprecipitate (n=3). Vertical bars, ± SEM; *, p<0.05. (C) XIAP, NEDD4 and NEDD4L were knocked down using siRNA in HEK293T, CTR1 KO-1 and CTR1 KO-2 cells and CTR2 protein levels were measured. The immunoblot shown is representative of 3 independent experiments.