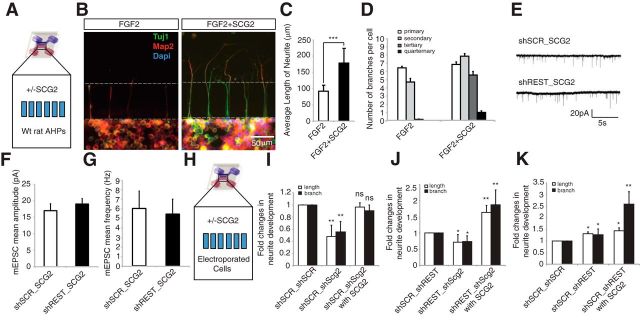
Figure 5.
Exogenous SCG2 induces differentiation and maturation of AHPs. A, Recombinant SCG2 was added in the top chamber (treatment chamber) of a microfluidic device; WT AHPs were plated in the bottom chamber (cell chamber). B, Effect of recombinant SCG2 treatment (20 ng/ml) on AHPs. Recombinant SCG2-treated or untreated AHPs were stained with anti-TUJ1 (green), anti-Map2 (red), and DAPI (blue). C, Treatment with SCG2 promoted significant neurite outgrowth. ***p < 0.0005. n = 80. D, SCG2 promoted neurite branching of wild-type AHPs. The number of neurite sub-branches increased upon treatment with recombinant SCG2. E, Example of mEPSC recordings taken from shSCR_SCG2- and shREST_SCG2-treated neurons. F, Treating shSCR primary neurons with SCG2 (10 ng/ml) was sufficient to increase mEPSC amplitude to a level comparable with that of shREST-transfected neurons (shSCR_SCG2: n = 13, mean 17.0 ± 2.1 pA; shREST_SCG2: n = 8, mean 19.0 ± 1.7 pA). G, SCG2 treatment also abolished the difference in mEPSC frequency between shSCR- and shREST-transfected neurons (shSCR_SCG2: mean 6.4 ± 2.0 Hz; shREST_SCG2: mean 5.8 ± 1.7 Hz). H, Schematic representation of the microfluidic device. I–K, Fold changes in neurite outgrowth (length and branches) upon different combinations of double knockdown. AHPs were transfected with two different constructs encoded by fluorescent colors (mCherry and GFP) for each of the combinatory double knockdown setups (shSCR/scSCR, shSCR/shScg2, shREST/shSCR, and shREST/shScg2) and then cultured in the presence of FGF2 for 5 d. *p < 0.05. **p < 0.005. ns, Not significant.