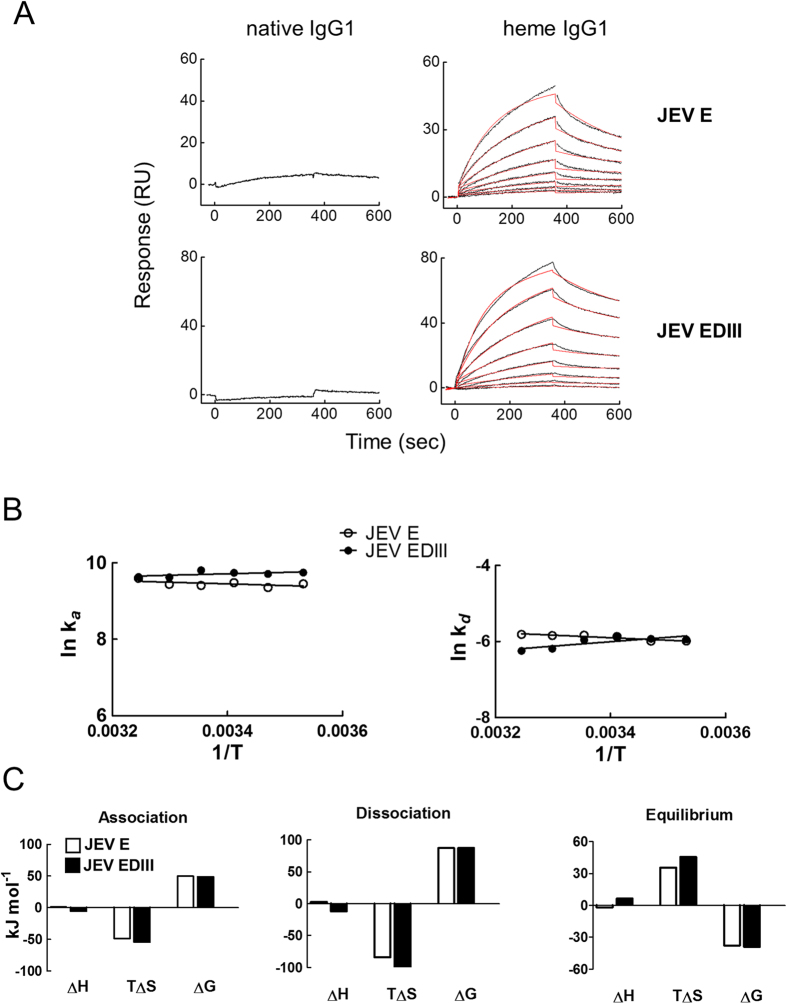
Figure 2. Kinetic and thermodynamic analyses of interaction of heme-exposed human IgG1 (mAb21) with JEV E and EDIII proteins.
(A) Real-time interaction profiles of binding of native or heme-exposed human monoclonal IgG1, mAb21 to immobilized recombinant JEV E and EDIII proteins. The real-time interaction profiles obtained after injection of native mAb21, diluted to 500 nM are presented in the left panels. The binding profiles of heme-exposed mAb21 at 500, 250, 125, 62.5, 31.25, 15.63, 7.81, and 3.90 nM are presented on the right panels. The binding analyses were performed at 25 °C. The graphs show experimentally determined binding curves (black lines) and curves generated by globally fitting the data by BIA evaluation software (red line). The estimated kinetic parameters are presented on Table 1. (B) Arrhenius plots showing the natural logarithm values of association and dissociation rate constants of the heme-sensitive mAb21 obtained after interaction with JEV E (open circles) and JEV EDIII (filled circles) as a function of reciprocal values of temperature (in Kelvins). To generate these plots the kinetic rate constants were determined by global analysis of sensorgrams generated after evaluation of binding kinetics of the heme-exposed mAb21 with immobilized JEV proteins at varying temperatures (10, 15, 20, 25, 30, and 35 °C). Linear regression analyses were applied to obtain the slopes of the temperature dependency. (C) Association, dissociation and equilibrium thermodynamic parameters of binding of heme-exposed mAb21 to JEV E and EDIII. Changes in the enthalpy, entropy and free energy during different phases of the interaction of heme-exposed mAb21 with JEV E (white bars) and EDIII (black bars) are depicted. The changes in non-equilibrium thermodynamic parameters were evaluated by applying Eyring’s analyses on the data from Arrhenius plots.