Abstract
Characterization/identification of the clusters/associates in liquids has long been a challenging topic. In this paper, we report a method to identify molecules with two different existing forms in a binary liquid solution. In this so-called two-state situation, the excess infrared spectra of a vibration mode of the respective molecule will show identical band shape if the other component is transparent in the region. More conveniently, the positions of the positive peak, negative peak, and zero-value will be seen to be fixed with varying compositions of the binary system. In the case of non-two-state mixtures, for example the mere solvation of solute by solvent, those positions will be variable. The conclusions are supported/demonstrated by computational simulation and experiments on two binary systems, D2O−H2O and C6F5I−cyclo-C6H12.
Liquids are much more complicated than gases and solids in the view point of theoretical treatment. They do not like gases which are typified by far molecular-separation and thus can be dealt with modified perfect gas law, nor do they like crystals which have definite structures and thus can be treated by the laws of solid-state physics. The biggest challenge in the study of liquids has been to describe their structures1.
The earliest models take liquids as totally disordered structures. Later on, it was found that liquids are long range-disordered and short range-ordered2. The presence of particular interactions such as hydrogen bonding3,4, halogen bonding5, and π–π stacking interactions6, will result in molecular association. It has been hypothesized that the associates could be dimer, trimer, tetramer, and multimers, either in linear, cyclic, or even branched forms7,8. If the associates are very stable and thus can be considered as discrete species, mass spectroscopy and methods to determine molecular weight such as freezing point depression can be of help. Unfortunately, most associates are not very stable, and can even be short-lived. To identify these not-very-stable associates, therefore, has been a long standing unsolved problem. In this paper, we report our effort to identify the associates using excess infrared spectroscopy.
Infrared spectra provide rich information of molecular structures. The absorption bands, however, are heavily overlapped for liquid samples. The concept of excess spectroscopy, proposed in our laboratory9,10, can improve the resolution to some extent. For example, by examining the O−H stretching band of tert-butanol–tetrachloride binary mixtures, we were able to identify four species in the mixtures, namely monomer, dimer, trimer, and multimers10. Other than this binary system, a number of binary systems including ionic liquids and molecular solvents have been investigated11,12,13. One interesting phenomenon is that the positions of some excess peaks are independent of concentration. As exemplified in Fig. 1(a,b), the excess peaks over the ν(O−H) in C2H5OH−CD3CN system (a) and ν(O−H) stretching in tert-butanol−CCl4 system (b) show fixed positions10,13.
Figure 1. Excess infrared spectra.
(a) O–H stretching in C2H5OH−CD3CN system13. (b) O–H stretching in tert-butanol–CCl4 system10. (c) C ≡ N stretching in CD3CN–CCl4 system. Mole fractions of the binary mixtures have been indicated by the respective curves in the figure.
It should be stressed that not all the excess peaks behave like this. The positions can be concentration-dependent as shown in Fig. 1(c) for ν(C ≡ N) stretching in CD3CN−CCl4 system. Intuitively, the fixed position of an excess band should mean something, most likely a reflection of identical configuration of the concerned bond, plus the same or close solvent environment to the respective vibrational mode. In this paper, we will show that this is true under certain conditions, particularly when there are only two existing forms of the concerned molecules. By changing the concentration of the mixtures, the amount of the two forms will change. This is regarded as a two-state transformation.
The significance of the present work lies also on its correlation to the in-situ identification of molecular clusters in liquids. Up to date, there have been some experimental methods to investigate molecular clusters14. But they are almost all for gas phase identification. For liquid state samples, for example water, their clusters have been studied by spraying the samples into inert solvent such as liquid helium15,16. Molecular clusters identified by these methods cannot reflect the real situations in liquids. Recently, Ben-Amotz and co-workers proposed that solvent molecules in a solution can be classified as bulk and solvation-shell molecules17,18,19. They used a multivariate curve fitting method to extract the spectrum of the latter. In principle, the idea can be used to extract the spectra of molecular clusters, while the detailed information of those clusters, including their types and spectroscopic properties, is required to be known. As a matter of fact, we have to accept that unambiguous in-situ identification of molecular clusters in pure liquids and liquid mixtures is an unsolved problem. The method proposed in this work gives a solution to the case when there are two existing forms of the target molecule in a binary system. The two forms can be two types molecular clusters or one type cluster plus monomer of the concerned component.
Results and Discussion
Theory
We consider a binary system. For each of the two components, the absorbance at a selected wavenumber is described in the form of Beer-Lambert Law:
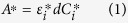 |
where εi* and Ci* are the molar absorption coefficient and the molarity of the compound i in pure form, redspectively, d the light path. In the case of the binary mixtures, we assume the form of Beer-Lambert Law is still applicable:
 |
where C = C1+C2. Generally speaking, absorption coefficient ε of the mixture is not a constant, but a function of wavelength and concentrations.
We define the absorption coefficient of an ideal mixture as:
 |
where x1 and x2 are mole fractions of the two components. Then, the excess infrared spectrum, in the form of excess absorption coefficient, is defined as follows:
 |
Now we purposely identify an absorption band of component 2 (solute, M), which is not overlapped with absorption bands from the other component (solvent, S). This means that the respective wavenumber range is transparent to the solvent. We assume that there are two existing forms of the solute, for example with and without forming complex with solvent (M1 and M2), over the entire or part of the concentration range. Their molar absorptivities are denoted as ε21 and ε22, which are independent on concentrations. The amounts of the two forms of the solute, however, are variable. The fraction of form 1 is denoted as δ over the two forms of the solute. In the absence of solvent, the fraction is δ0. With these parameters, Equation (3) turns to be
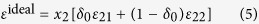 |
Molar absorptivity of the real binary mixture is,
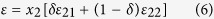 |
Then, based on Equation (4), excess molar absorptivity of a real binary mixture is,
 |
It is thus clear that the term  defines the shape of an excess spectrum band, which is independent of the apparent concentration of the binary system. Only when the molar absorptivities of the two forms of the solute equal to each other, the excess function can be zero. Thus we conclude that the peak positions and zero-intensity frequency of the excess spectroscopic bands are all fixed in a series of binary mixtures, if there are only two existing forms of the solute and the solvent is transparent over the examined wavenumber range.
defines the shape of an excess spectrum band, which is independent of the apparent concentration of the binary system. Only when the molar absorptivities of the two forms of the solute equal to each other, the excess function can be zero. Thus we conclude that the peak positions and zero-intensity frequency of the excess spectroscopic bands are all fixed in a series of binary mixtures, if there are only two existing forms of the solute and the solvent is transparent over the examined wavenumber range.
Digital simulation
With the above theoretical analysis, we have performed some digital simulation of excess infrared spectra to show the ideal appearance of the excess infrared spectra. Here, a single absorption band is expressed by the Lorentzian function as follows,
 |
where w represents the full width at half-height, ν0 the center position of band, and ε0, the molar absorptivity when ν = ν0. When there are two forms of the solute molecules in the binary system, the spectral function is expressed as follows,
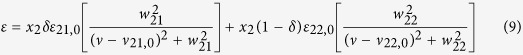 |
The two peak positions are arbitrarily set at ν21,0 = 3275 cm−1 (State 1) and ν22,0 = 3305 cm−1 (State 2). By assigning the following parameters: ε21,0 = 1, ε22,0 = 0.9, w21 = 80, w22 = 70, the absorption bands representing the two states of the molecules can be derived and the results are shown in the inset of Fig. 2(a). Assuming pure M only exists in the form of State 1, and when the mole fraction of M decreases, the fraction of State 1 in M also decreases in the M−S binary system. Thus giving a series of apparent concentration x2 =1, 0.8, 0.6, 0.4, 0.2, and 0, we assume δ = 1, 0.7, 0.45, 0.25, 0.1 and 0. IR spectra and excess infrared spectra are simulated and the results are shown in Fig. 2. As can be seen in Fig. 2(a), the absorption peak looks like singular and it shifts gradually to higher wavenumber with increasing solvent concentration. Apparently, the blue shift would have been caused by solvation effect.
Figure 2.
Simulated infrared spectra (a,c) and the respective excess spectra (b,d) for the two-state transformation system. The dashed and dash-dot dotted lines in (a,c) depict spectra of pure M and S. The inserts are: the absorption bands representing the two existing forms of the molecules in their pure states (a,c), overlapping presentation of the excess curves (b,d).
The excess infrared spectra shown in Fig. 2(b) provide correct insights of the system. Apart from showing a positive peak at the higher wavenumber side and a negative peak at the lower wavenumber side in each curve, which is the same with solvation effect, the spectra give fixed positions of positive and negative peaks at 3314 and 3264 cm−1, respectively. Meanwhile, the zero-value points of the four excess spectra appear at identical position. Different values of the parameters generate excess infrared spectra with different shapes. When there is significant difference between the two w values, namely one broad peak overlaps with a very narrow peak, two zero-value points can be seen. Shown in Fig. 2(c,d) is one such example where ε21,0 = 1, ε22,0 = 1.4, w21 = 200, w22 = 90. Even in this case, the positive and negative peak positions and the zero-value positions are all fixed.
We also examined the solvation effect, where an infrared absorption band of component 2 shifts gradually. To do the simulation, we first constructed an absorption peak of component 2 centered at 3275 cm−1 with ε2,0 = 1 and half peak width w2 = 80. Then the mole fraction of the component 2 decreases by 0.2 each step, and at the same time the absorption peak shifts to higher wavenumber by 10 cm−1. The Lorentzian peak is described as follows
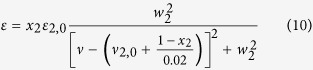 |
The simulated infrared spectra as shown in Fig. 3(a) are quite similar to those in Fig. 2(a). The simulated excess infrared spectra, however, show completely different results. The positions of both positive and negative peaks, as well as the position of the zero-value point, are not fixed.
Figure 3.
Simulated infrared spectra (a) and excess infrared spectra (b) in the presence of solvation effect. The dashed and dash-dot dotted lines in (a) depict spectra of pure M and S, respectively. The insert is the overlapping presentation of the excess curves.
If two distinct states and solvent effects are combined, or there are more than two distinct states of the solute, the Equation (7) will not hold anymore. More or less, the positions of the positive and negative peaks will subject to changes. Nevertheless, our simulation indicates that, depending on the extent of hydration or the relative separation of the multi-state peaks, positions of part or all of the peaks in the excess spectra can be relatively fixed (see Supplementary Information). We, therefore, conclude that if the position of an excess peak is relatively fixed, most likely it represents a distinct species.
D2O−H2O system
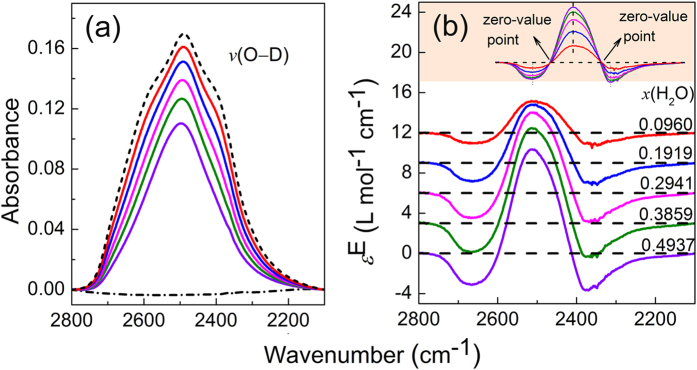
The hydrogen/deuterium atoms in a water molecule are not bonded to one molecule forever, but rather they can exchange with the hydrogen/deuterium in the neighboring molecules. Consequently, the H and D atoms in D2O−H2O mixture will switch one another to form the third species, HDO20,21. In D2O−H2O binary system, there are two kinds of O−D, those in HDO and in D2O. This is the case of two-state transformation upon changing concentration.
The partial ATR-FTIR and excess spectra of D2O−H2O system in the O−D stretching region are shown in Fig. 4. Normal water has no absorptions in the wavenumber region. The absorption band in Fig. 4(a) is assigned to ν(O−D) according to literature20. In Fig. 4(b), the excess spectra show two fixed-position negative peaks, one fixed positive peak, and two fixed zero-value points, which are the same as the simulation result shown in Fig. 2(d). This demonstrates that, in the binary system with two-state transformation, both the excess peaks and zero-value points are fixed.
Figure 4.
Infrared spectra (a) and the respective excess infrared spectra (b) of D2O–H2O system in the range of the O–D stretching vibration. The dashed and dash-dot dotted lines in (a) depict spectra of pure D2O and H2O, respectively.
C6F5I− cyclo-C6H12 system
The C6F5I–cyclo-C6H12 system was also studied by ATR-FTIR technique. The IR and excess infrared spectra of the C6F5I–cyclo-C6H12 system in the C–I stretching region are shown in Fig. 5. The band at 805 cm−1 is attributed to the C−I stretching vibration22. In Fig. 5(a), from top to bottom, the peak position is blue-shifted. In Fig. 5(b), the excess spectra show fixed positions of positive peak, negative peak, and zero-value points, which are in consistent with the simulation results shown in Fig. 2(b). This strongly suggests that C6F5I–cyclo-C6H12 system exists in two distinct states upon dilution with cyclo-C6H12. For C6F5I, cyclo-C6H12 is an inert solvent. So the two states may be attributed to the absorptions of monomer and self-aggregated C6F5I12. Most likely, the self-aggregated C6F5I is the dimer of the molecule. Quantum chemical calculations were performed to find the optimized structure of the C6F5I dimer, which is shown in the inset of Fig. 5(a). As can be seen, the two molecules form a displaced π−π stacking interaction pair. The distance of the two planes is 0.303 nm. The interaction energy is −100.25 kJ/mol, suggesting the dimer is quite stable. Furthermore, the calculated frequencies of v(C−I) indicate that there is a redshift upon formation of dimer. Thus the negative peak at lower wavenumber is assigned to dimer and the positive one to monomer.
Figure 5.
Infrared spectra (a) and excess infrared spectra (b) of the C6F5I–cyclo-C6H12 system at different mole fraction of C6F5I in the wavenumber range of the C–I stretching vibration. The dashed and dash-dot dotted lines in (a) depict spectra of pure C6F5I and cyclo-C6H12, respectively.
Conclusions
In this paper, we developed a method to differentiate two-state and non-two-state of molecular exiting forms in binary liquid mixtures, based on the excess spectroscopic technique. If a molecule exists in two forms over a range of concentration in a binary mixture, the excess band of a vibration mode of the molecule will show fixed peak positions and zero-intensity frequencies in the concentration range if the other component has no absorption in the region. Otherwise, these positions should be concentration-dependent. These features are demonstrated with numerical simulation and experiments. In D2O−H2O system, there are two O−D stretching forms. They are readily assigned to that from D2O and DOH. In C6F5I–cyclo-C6H12 system, the excess infrared spectra in the C−I stretching region suggest that there are two forms of C6F5I during the mixing process. They are most likely self-aggregated and non-aggregated C6F5I. The aggregation is believed to be in the form of displaced π−π stacking. For more complicated situations such as the coexistence of three or more forms of the concerned component, the feature/shape of the excess spectra will be concentration-dependent. But if the position of a positive peak or negative peak in a series of excess spectra is relatively fixed, most likely it indicates a distinct species.
Methods
Chemicals
D2O (>99%) was purchased from Armar Chemicals. C6F5I (>98%) was purchased from J&K Scientific. Cyclo-C6H12 (>99%) was from Beijing Chemical Plant (Beijing, China). The samples were used without further purification.
Sample preparation
A series of D2O−H2O and C6F5I–cyclo-C6H12 binary mixtures were prepared by weighing. The mole fractions of H2O in D2O−H2O mixtures are 1, 0.4937, 0.3859, 0.2941, 0.1919, 0.0960, and 0. The mole fractions of C6F5I in C6F5I–cyclo-C6H12 mixtures are 1, 0.8815, 0.7928, 0.7073, 0.5996, 0.5027, 0.3888, 0.2918, 0.2044, 0.1050, and 0.
FTIR spectroscopy
FTIR spectra over the range from 4000 to 650 cm−1 were collected at room temperature (~25 °C) using a Nicolet 5700 FTIR spectrometer, equipped with an MCT detector. Two ATR cells made of trapezoidal ZnSe/Ge crystals were used in the experiments. A ZnSe crystal with an incident angle of 45° and 12 reflections was used to examine the stretching bands of O−D in D2O−H2O system. A Ge crystal with an angle of 60° and 7 reflections was used to examine the strong stretching bands of C−I in C6F5I–cyclo-C6H12 system. Spectra were recorded with a resolution of 2 cm−1, a zero filling factor of 2, and 32 parallel scans. The refractive indexes of solutions were measured with a refractometer at 25 °C. The formulas suggested by Hansen23, were used to do the ATR corrections.
Excess infrared spectroscopy
The theory of excess infrared spectroscopy has been described in detail elsewhere9,10. Briefly, an excess infrared spectrum is defined as the difference between the spectrum of a real solution and that of the respective ideal solution under identical conditions. The working equation in calculating the excess infrared spectrum is as follows:
 |
where A is the absorbance of the mixture, d is the light path length, C1 and C2 are molarities of the two components, x1 and x2 are mole fractions of components 1 and 2, and ε1* and ε2* are molar absorption coefficients of the two components in their pure states, respectively. The calculation of the excess infrared spectra was programmed using Matlab 7.0 (Math Works Inc., Natick, MA).
Quantum chemical calculations
Quantum chemical calculations were performed by employing second-order many-body perturbation theory (MP2) at mixed basis sets with the Gaussian 09 program24. The molecular geometry were fully optimized with the LANL2DZ25, basis set for iodine atom and the aug-cc-pVDZ26, basis set for hydrogen, carbon, and fluorine atoms. All the optimized geometries were recognized as local minima with no imaginary frequency.
Additional Information
How to cite this article: Zhou, Y. et al. Two-State or Non-Two-State? An Excess Spectroscopy-based Approach to Differentiate the Existing Forms of Molecules in Liquids Mixtures. Sci. Rep. 5, 16379; doi: 10.1038/srep16379 (2015).
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant Nos. 21133009 & 21473099).
Footnotes
Author Contributions Y.Z., Y.Z.Z., H.Y.S. and Z.W.Y. designed the study; Y.Z. conducted the experiments; Y.Z. and Y.Z.Z. analyzed the data; Z.Y., H.Y.S. and G.D. contributed the theoretical derivation; Z.Y. and Z.W.Y. interpreted the results and wrote the manuscript.
References
- Reichardt C. & Welton T. Solvents and Solvent Effects in Organic Chemistry 4th edn. Ch. 1, 1–6 (John Wiley & Sons, 2011). [Google Scholar]
- Ise N. & Okubo T. Ordered distribution of electrically charged solutes in dilute solutions. Acc. Chem. Res. 13, 303–309 (1980). [Google Scholar]
- Gadre S. R., Yeole S. D. & Sahu N. Quantum chemical investigations on molecular clusters. Chem. Rev. 114, 12132–12173 (2014). [DOI] [PubMed] [Google Scholar]
- Guo J. H. et al. Molecular structure of alcohol-water mixtures. Phys. Rev. Lett. 91, 157401 (2003). [DOI] [PubMed] [Google Scholar]
- Metrangolo P., Neukirch H., Pilati T. & Resnati G. Halogen bonding based recognition processes: A world parallel to hydrogen bonding. Acc. Chem. Res. 38, 386–395 (2005). [DOI] [PubMed] [Google Scholar]
- Sharma B., Srivastava H. K., Gayatri G. & Sastry G. N. Energy decomposition analysis of cation–π, metal ion–lone pair, hydrogen bonded, charge-assisted hydrogen-bonded, and π–π interactions. J. Comput. Chem. 36, 529–538 (2015). [DOI] [PubMed] [Google Scholar]
- Vaitheeswaran S., Yin H., Rasaiah J. C. & Hummer G. Water clusters in nonpolar cavities. Proc. Natl. Acad. Sci. USA 101, 17002–17005 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally R. J. & Blake G. A. Molecular interactions and hydrogen bond tunneling dynamics: some new perspectives. Science 259, 1570–1575 (1993). [DOI] [PubMed] [Google Scholar]
- Li Q. Z., Wu G. S. & Yu Z. W. The role of methyl groups in the formation of hydrogen bond in DMSO-methanol mixtures J. Am. Chem. Soc. 128, 1438–1439 (2006). [DOI] [PubMed] [Google Scholar]
- Li Q. Z., Wang N. N., Zhou Q., Sun S. Q. & Yu Z. W. Excess infrared absorption spectroscopy and its applications in the studies of hydrogen bonds in alcohol-containing binary mixtures. Appl. Spectrosc. 62, 166–170 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang Q. G., Wang N. N. & Yu Z. W. Hydrogen bonding interactions between the ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate and water. J. Phys. Chem. B 114, 4747–4754 (2010). [DOI] [PubMed] [Google Scholar]
- Zheng Y. Z., Deng G., Zhou Y., Sun H. Y. & Yu Z. W. A Comparative study of halogen-bond and hydrogen-bond interactions between benzene derivatives and dimethyl sulphoxide. Chem Phys Chem 16, 2594–2601 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zheng Y. Z., Sun H. Y., Deng G. & Yu Z. W. Hydrogen bonding interactions in ethanol and acetonitrile binary system: A near and mid-infrared spectroscopic study. J. Mol. Struct. 1069, 251–257 (2014). [Google Scholar]
- Ludwig R. Water: From clusters to the bulk. Angew. Chem. Int. Ed. 40, 1808–1827 (2001) [PubMed] [Google Scholar]
- Nauta K. & Miller R. E. Formation of cyclic water hexamer in liquid helium: The smallest piece of ice. Science 287, 293–295 (2000). [DOI] [PubMed] [Google Scholar]
- Mir M. H. & Vittal J. J. Phase transition accompanied by transformation of an elusive discrete cyclic water heptamer to a bicyclic (H2O)7 cluster. Angew. Chem. Int. Ed. 46, 5925–5928 (2007). [DOI] [PubMed] [Google Scholar]
- Fega K. R., Wilcox D. S. & Ben-Amotz D. Application of Raman multivariate curve resolution to solvation-shell spectroscopy. Appl. Spectrosc. 66, 282–288 (2012). [DOI] [PubMed] [Google Scholar]
- Perera P., Wyche M., Loethen Y. & Ben-Amotz D. Solute-induced perturbations of solvent-shell molecules observed using multivariate Raman curve resolution. J. Am. Chem. Soc. 130, 4576–4577 (2008). [DOI] [PubMed] [Google Scholar]
- Davis J. G., Gierszal K. P., Wang P. & Ben-Amotz D. Water structural transformation at molecular hydrophobic interfaces. Nature 491, 582–585 (2012). [DOI] [PubMed] [Google Scholar]
- Maxa J. J. & Chapadosb C. Isotope effects in liquid water by infrared spectroscopy. J. Chem. Phys. 116, 4626–4642 (2002). [Google Scholar]
- Saihara K., Yoshimura Y., Ohta S. & Shimizu A. Properties of water confined in ionic liquids. Sci. Rep. 5, 10619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankiss S. & Harrison D. Thermodynamic properties of fluorine compounds—XVI. The vibrational spectra and thermodynamic functions of pentafluorobenzene, chloropentafluorobenzene, bromopentafluorobenzene and methylpentafluorobenzene. Spectrochimica Acta Part A: Mol. Spectrosc. 31, 1839–1864 (1975). [Google Scholar]
- Hansen W. N. Expanded Formulas for Attenuated Total Reflection and the Derivation of Absorption Rules for Single and Multiple ATR Spectrometer Cells. Spectrochim. Acta 21, 815–833 (1965). [Google Scholar]
- Frisch M. J. et al. Gaussian 09, Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- Wadt W. R. & Hay P. J. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270 (1985). [Google Scholar]
- Dunning T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007 (1989). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.