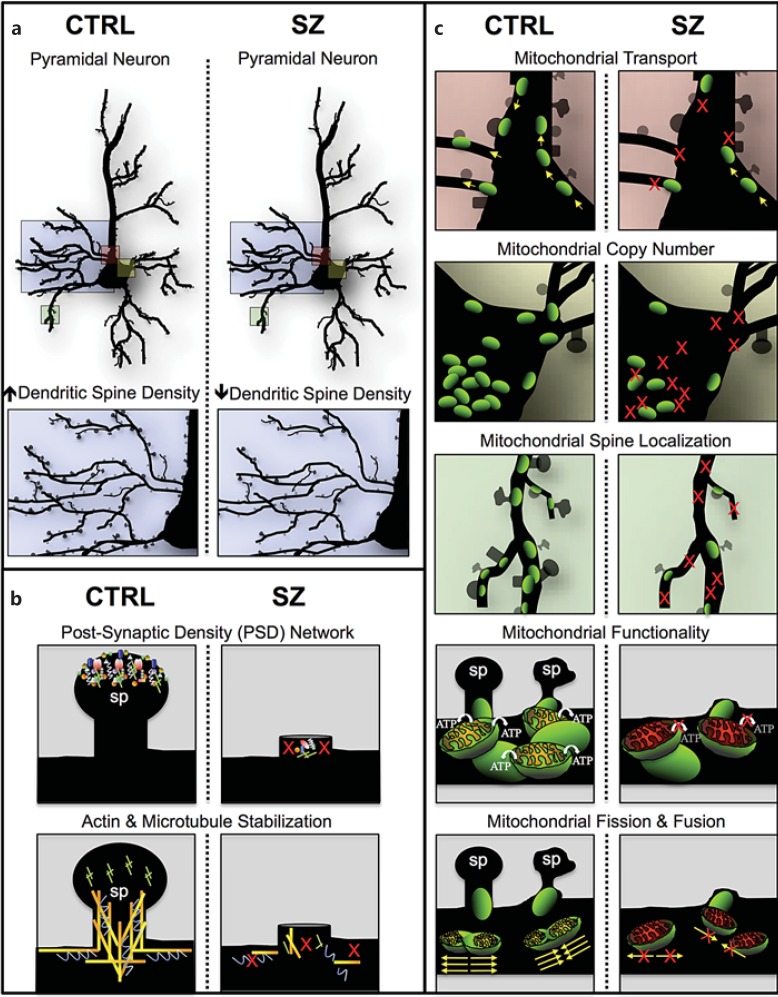
Fig. 1.
Multifactorial model of mitochondrial dysfunction in SZ. Cartoon illustration of our hypothesis of a multifactorial model where alterations in gene networks specific to PSD, cytoskeleton organization, and an array of mitochondrial processes may independently (or additively) lead to dendritic spine deficits, SZ symptom onset and polygenic disease risk. a Pyramidal neurons in control (CTRL) and SZ brains displaying a reduction in dendritic spine density in SZ pathology (blue box) and neuronal regions potentially affected by mitochondrial dysfunction (pink, yellow and green boxes). Colors refer to the online version only. b Dendritic spines (sp) in CTRL brains and spine loss in SZ brains due to polygenic variants in nonmitochondrial networks that have been implicated in SZ etiology (i.e., postsynaptic density and cytoskeleton organization). c Neuronal regions potentially affected by a variety of mitochondrial processes (i.e., transport, copy number, spine localization, functionality, and fusion/fission) and their possible role in dendritic spine loss in SZ brains compared to CTRL. We hypothesize that a signature of mitochondrial dysfunction can occur in SZ independently or additively in b and c. In b, mitochondrial deficits are a direct result of spine loss due to the polygenic burden in nonmitochondrial pathways like the PSD and cytoskeleton networks (i.e., PSD/cytoskeleton gene aberrations → spine loss ← → mitochondrial dysfunction); in c, mitochondrial deficits are a direct result of aberrations in nuclear genes with mitochondrial function and/or in the mitochondrial genome, and there is also spine loss-induced mitochondria loss (i.e., mitochondrial gene aberrations → mitochondrial dysfunction ← → spine loss); in c the mitochondria loss of function is primary, while in b the spine loss is the primary causative event.