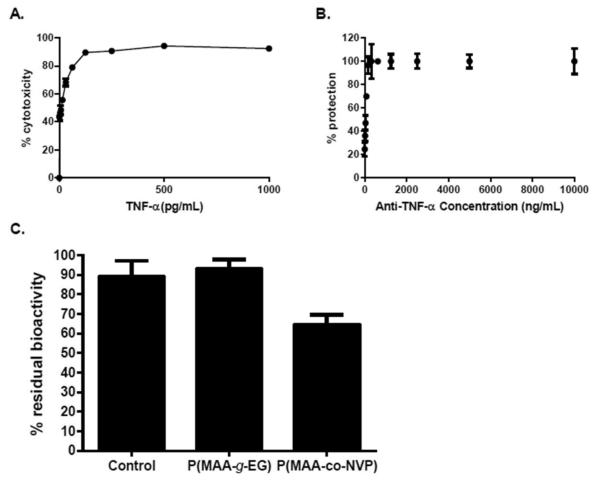
Figure 3.
Anti-TNF-α antibody in vitro neutralization activity is maintained upon release from P(MAA-g-EG) microparticles. Optimization of TNF-α cytotoxic dose and anti-TNF-α antibody neutralization dose. A 500 pg/mL dose of TNF-α was suffcient to achieve around 95% of cytotoxicity in L929 cells, while 5 μg/mL of anti-TNF-α antibody was necessary to protect close to 100% of cells. (A) Titration of cytolytic activity of the recombinant TNF-α. (B) Neutralization of TNF-α by different doses of antibod. The viability of the cells was quantified by MTS assay and represented as percentages against control cells cultured in the absence of TNF-α. Error bars represents standard deviation of three independent experiments performed in triplicate. (C) Anti-TNF-α-loaded P(MAA-g-EG) microparticles are capable of releasing bioactive antibody, while loading and release from P(MAA-co-NVP) microparticles affects antibody bioactivity. Percentage residual bioactivity from TNF-α cytolytic activity achieved by anti-TNF-α antibody. L929 cells were cultured for 24 h in the presence of TNF-α and unencapsulated antibody, or antibodies released from microparticles. The viability of the cells was quantified by the MTS assay and represented as percentages against control cells cultured in the absence of TNF-α. Error bars indicate standard error of three independent experiments performed in triplicate.