Figure 5.
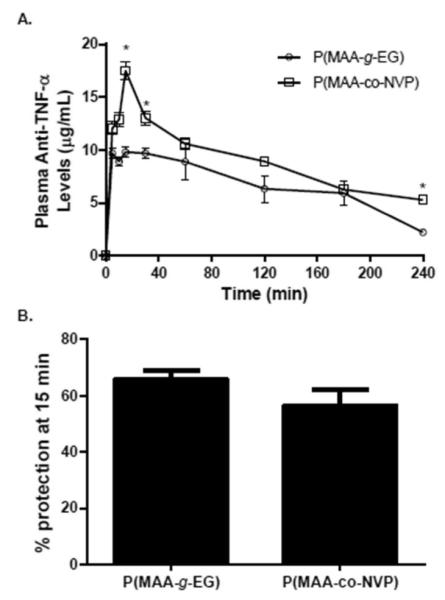
Bioavailability and ex-vivo bioactivity of anti-TNF-α antibody upon release from hydrogel microparticles. (A) Plasma anti-TNF-α levels versus time profiles following direct injection of anti-TNF-α-loaded P(MAA-g-EG) microparticles (n = 6) and anti-TNF-α-loaded P(MAA-co-NVP) microparticles (n = 6) into an intestinal closed-loop in healthy adult Sprague-Dawley rats. Blood samples were then taken at 5, 10, 15, 30, 60, 120, 180, and 240 min. The dose of anti-TNF-α loaded into microparticles was 70 μg/kg body weight. Anti-TNF-α mAb concentration in diluted serum was measured by ELISA (B) L929 cell TNF-α neutralization bioassay was utilized to assess the functionality of the released anti-TNF-α antibody in serum taken 15 min after hydrogel formulation injection into the intestinal loop. Concentration and % protection are presented as mean ± SEM The asterisk (*) represents a statistically significant difference from all other treatments at p ≤ 0.05.
