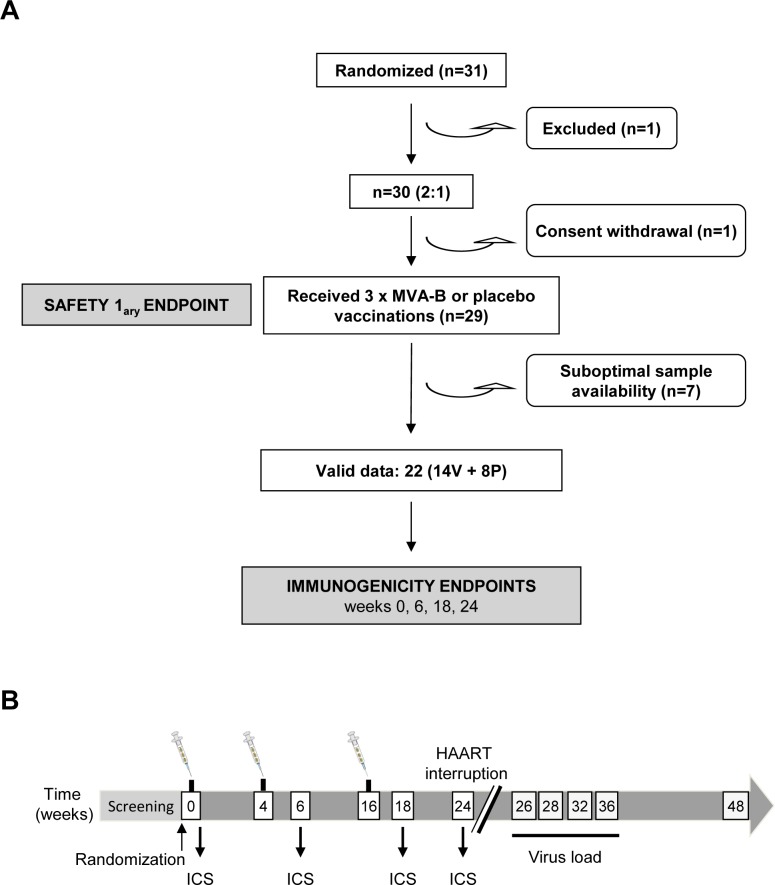
Fig 1. Flow chart of the RISVAC03 study design and distribution of volunteers.
(A) A total of 31 volunteers were randomized (21 to the MVA-B arm and 10 to the placebo arm) but one was excluded. One volunteer withdrew consent and 29 volunteers received three doses of the vaccine or placebo. Seven patients had suboptimal sample availability so the immunogenicity to vaccination or placebo was assessed by ICS in 22 volunteers. All 29 participants were included in the safety analysis. V: vaccine; P: placebo. (B) Chronological diagram showing the vaccination schedule, the HAART interruption timeline, the immunogenicity endpoints and the determination of HIV-1 viral loads.