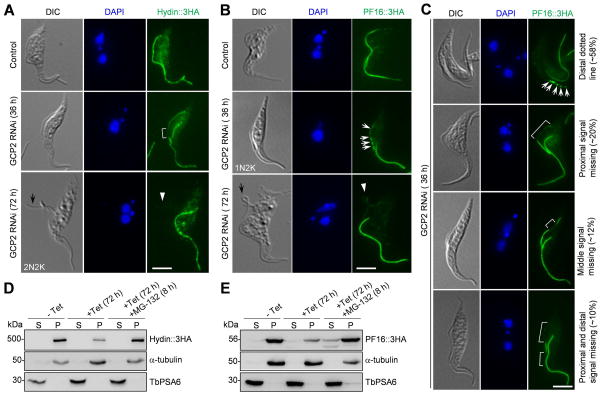
Figure 6. GCP2 RNAi disrupts Hydin and PF16 assembly to the new flagellum.
(A, B). Effect of GCP2 RNAi on the localization of Hydin (A) and PF16 (B). Hydin and PF16 were each endogenously tagged with a triple HA epitope in cells harboring the GCP2 RNAi construct. Non-induced control and GCP2 RNAi-induced cells (36 h and 72 h) were immunostained with anti-HA antibody to detect Hydin::3HA and PF16::3HA. The white bracket shows the reduced Hydin::3HA signal in the middle portion of the new flagellum, the white arrows show the portions of the new flagellum with reduced PF16::3HA signal, and the white arrowheads indicate the signal of Hydin::3HA and PF16::3HA in the detached flagellum (black arrows). Scale bars: 5 μm. (C). GCP2 RNAi caused random depletion of PF16::3HA from the new flagellum at earlier time points of RNAi induction. White arrows show the PF16::3HA signal (as multiple dots) at the distal portion of the new flagellum, and white brackets outline the portions of the new flagellum with much reduced PF16::3HA signal. Scale bar: 5 μm. (D, E). Effect of GCP2 RNAi on the stability of Hydin (D) and PF16 (E) in cytosolic and the cytoskeletal fractions. Non-induced control and GCP2 RNAi cells treated without and with MG-132 were lysed in PEME buffer containing 1% NP-40 for cytoskeleton preparation. The soluble cytosolic fraction (S) and cytoskeletal pellet fraction (P) were separated by centrifugation, loaded onto a SDS–PAGE gel and transferred onto a PVDF membrane for western blotting with anti-HA antibody to detect Hydin::3HA and PF16::3HA. The same blot was probed with anti-TbPSA6 as the cytosolic marker and with anti-α-tubulin as the cytoskeletal marker. Similar results were obtained in three independent experiments.