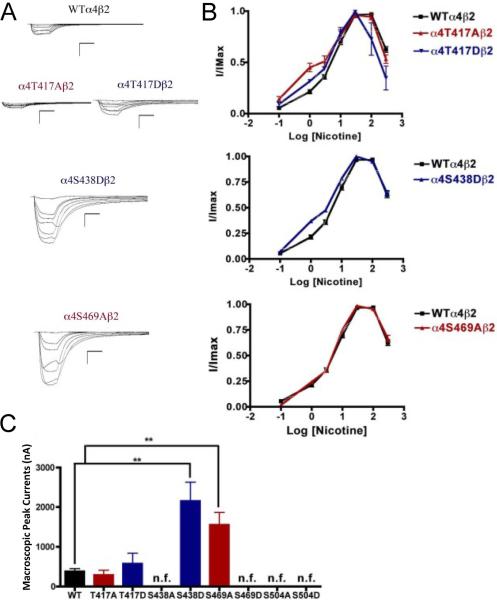
Fig. 4.
Functional characterization of mutations on CKII putative phosphorylation sites in the α4 subunit with nicotine used as agonist. Voltage-clamp recordings were used to determine the macroscopic response to several nicotine concentrations of mutations of CKII putative sites and wild-type α4β2 nAChRs expressed in Xenopus laevis oocytes. (A) Family of nicotine-induced macroscopic currents for mutations α4T417Aβ2, α4T417Dβ2, α4S438Dβ2, α4S469Aβ2, and the wild-type α4β2 nAChR. Calibration bars are shown for all family of currents, horizontal bars indicate time (5 s) and vertical bars indicate the inward current (500 nA). (B) Dose-response relationships obtained by voltage-clamp experiments with nicotine used as an agonist. Nicotine dose-response curves were determined using seven nicotine concentrations (0.1, 1, 3, 10, 30, 100, and 300 μM). The responses were normalized to the maximum response (I/Imax). (C) Comparison of the nicotine-induced macroscopic peak currents of the mutations and wild-type receptor, shown in nA (n=6-17) (**p<0.005).