Abstract
Purpose
To measure depressive symptoms in dry eye disease (DED) patients and controls using the Beck Depression Inventory (BDI) and determine the association between depressive and DED symptoms.
Methods
Fifty-three DED patients and 41 controls were recruited to the study. DED symptoms were assessed using the Symptom Burden Tool and the Ocular Surface Disease Index (OSDI) tool. Depressive symptoms were assessed using the BDI. Regression diagnostics were performed to detect outliers. Linear statistical models and polynomial regression were used to determine relationship between depressive symptoms and DED symptoms. Independent t-test was performed to determine differences in BDI scores between cases and controls. Scatter plots were generated and linear regression was used to estimate the association between scores. Logistic regression was used for the DED dichotomous outcome and depression status as exposure.
Results
Regression models revealed that the association is linear more than quadratic or cubic. After adjusting for age, gender, race, and psychiatric medication, the regression coefficient between DED symptom and depressive symptoms among DED cases was 1.22 (95% CI 0.27, 2.18). DED symptom scores and depression scores were statistically significantly different between DED cases and controls. Adjusted logistic regression revealed an OR of 2.79, 95% CI (0.96–8.12).
Conclusions
This study provides further evidence regarding the association between DED and depression and their symptoms. Prospective studies are needed to understand the mechanisms underlying the association between symptoms of depression and symptoms of DED.
INTRODUCTION
The aggressiveness of Dry Eye Disease (DED) treatment is based on the severity of symptoms. DED symptoms include pain, dryness, grittiness, itchiness, redness, burning or stinging, foreign body sensation, and light sensitivity. It is known that DED symptoms do not correlate with clinical signs, where certain clinical tests such as the Schirmer test do not correlate with patient reported dryness1. However, the chronic discomfort observed in DED patients may be associated with a decrease with the quality of life correlating it with affective interference2.
Recent case-control and cross-sectional studies have reported an association between depression and DED, post-traumatic stress disorder and DED, and anxiety and DED3–9. Examination of the association between depression and DED started when some researchers revealed that depressive mood is one of the underlying causes of subjective dry mouth10,11. Others suggested that dry eye symptoms and mood status may influence each other12. The 2007 Dry Eye Workshop (DEWS) report included a discussion on the debilitating symptoms of DED and their result in both psychological and physical effects that impact the quality of life13. Subsequently, Li et al assessed vision-related quality of life and psychosocial impacts and found correlations with depression and anxiety14. Labbe et al, using subjects from the Beijing Eye Study, showed that depression was associated with DED in particular with dry eye symptoms8.
We hypothesize that the presence of depression in DED may cause patients to perceive symptoms in an anomalous fashion compared to patients without depression. This is similar to the relationship between psychological and psycho-physiological characteristics with fibromyalgia15. This means that if depression was treated independently and its contribution to patient dry eye symptoms removed from the equation, then it may be possible to manage DED with less aggressive treatments (i.e. the frequency of medication intake and type of medication).
In this case-control study, we used the Beck Depression Inventory (BDI) to measure depressive symptoms in DED patients and controls to determine the association between depressive and DED symptoms.
METHODS
Study Overview
Study approval was obtained from the Institutional Review Board of the University of Illinois at Chicago. Symptomatic patients with DED were enrolled and written informed consent was obtained from all patients after the nature and possible consequences of research were explained. Research was conducted in accordance with the requirements of the Health Insurance Portability and Accountability Act and tenets of the Declaration of Helsinki. Eligible patients completed two DED symptom questionnaires and the BDI questionnaire to measure depressive symptoms. The DED symptom questionnaires were completed by the interviewer and the BDI questionnaire was completed by self-administration. Socio-demographic data and psychological and medication history were obtained by chart review. All subjects included in our study were > 18 years of age.
Study Population
Fifty-three patients were recruited from our dry eye clinic at the Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago. The DED sample included newly-diagnosed and established patients of DED who visited our clinic between November 2012 and June 2014. The diagnostic criteria as also mentioned in the DEWS report were: (i) a reporting of symptoms: burning sensation, irritation, grittiness or foreign body sensation, light sensitivity, pain, dryness, soreness, or discomfort in the eye; (ii) a Schirmer value of < 10mm/5min in either eye using Whatman filter strips #41 (Haag-Streit, Essex, UK) ; or (iii) positive corneal staining and/or Rose Bengal corneal and conjunctival staining of ≥ 1. The sampling method intended a sample of all DED patients attending the clinic between the dates mentioned above. However, given that this subjects are asked whether they would like to participate in this study, the final sample is characterized as a convenience sample.
Forty-one control patients who visited our general eye clinic with refraction-related complaints were recruited to the study. The inclusion criteria included no clinical diagnosis of DED, a Schirmer value of ≥ 10mm/5min, and no corneal staining. None of the control subjects enrolled were using tear supplements. Sampling was similar to cases, where an "all sample" technique was intended, however taking into consideration the willingness to participate characterizes this as more of a convenient sample.
Assessment of Depressive Symptoms and History of Depression
Depressive symptoms were measured using the BDI. The BDI is a widely used tool for measuring the severity of depressive symptoms16. The tool is a 21-question multiple-choice self-report inventory that was created by Aaron T. Beck and first published in 196117. The items for the BDI cover emotional, behavioral, and somatic symptoms. Beck, Steer, and Grabin concluded that reviews of factors analyses have identified three factors: Negative attitude toward self, performance impairment, and somatic disturbances18. The standard cutoff points for the BDI are 0–9: indicating minimal depression; 10–18: indicating mild depression; 19–29: indicating moderate depression; 30–63: indicating severe depression. In this study, the BDI score was divided into ≤ 9 or > 9. Depression status was determined as a composite variable through chart review. The variable was determined as "ever having depression" through medical and psychological history and/or through any documented history of prescribed psychiatric medications. This composite variable was determined because for some patients a diagnosis of clinical depression in their charts was not indicated; however psychiatric medication was listed among their medications. Psychiatric medication was also determined as a separate variable.
Assessment of DED Symptoms
DED symptoms were assessed using two tools: The Symptom Burden Tool2 and the Ocular Surface Disease Index (OSDI) tool19. The Symptom Burden Tool assesses four domains: persistence, intensity, activity, and affective interference. The OSDI tool measures persistence, activity interference, and environmental triggers. Scores on the OSDI range from 0–100 and from 0–520 for the Symptom Burden Tool. The use of the two tools is complementary. The OSDI is the most frequently used survey instrument for the assessment of ocular surface disease and its severity in DED research. It has demonstrated discriminant and concurrent validity and been shown effective for discriminating people with varying levels of DED19, 20. The Symptom Burden Tool is a recent tool that provides a more comprehensive symptom assessment using four domains (persistence, intensity, activity, and affective) adapted from chronic diseases with pain2.
Statistical Analysis
Histograms with the normal curve, Q-Q plots, and the statistical tests for normality were used to determine if the data is normally distributed. Demographic data were summarized as means ± standard deviation (SD) and percent distribution. For data representation and clinical interpretation, OSDI scores, DED symptom burden questionnaire scores, and BDI scores were summarized as mean ± standard deviation (SD). Regression diagnostics (studentized residuals and leverage) were performed to detect any outliers and unusual influential data. Scatter plots were generated with fitted lines between DED symptoms and depression symptoms. Linear statistical models and polynomial regression were run to determine the type of relationship between depressive symptoms and DED symptoms.
Independent t-test was performed to determine differences of BDI scores between cases and controls. Similarly, independent t-test comparison of DED symptom scores among cases diagnosed with depression and those without depression was also performed. Linear regression was used to estimate the association between DED symptom continuous scores and BDI depression scores. Logistic regression was used for the DED dichotomous outcome and depression status as exposure. Unadjusted and adjusted regression analysis was performed. Chi-square and Fisher's exact tests were used for categorical variables. Medians were also calculated for symptom scoring variables. Statistical significance was set at 0.05. Data were analyzed using STATA/SE v12 software.
RESULTS
Demographics
Table 1 shows the demographic distribution for case and control subjects. Regression diagnostics revealed three major outliers that influenced the data. These three cases had extreme scores of OSDI (one patient had an OSDI of 97.7) or BDI (two patients had a BDI of more than 30) were excluded from the analysis, making the total sample equal to 91 (50 cases and 41 controls). Mean age was comparable between the two groups 52.6 for DED cases and 50.0 for controls (P=0.43). In total, there were 32 males (16 DED case and 16 controls) and 62 females (37 DED cases and 25 controls) (P=0.39). The distribution for race was different between white and non-white. Among DED cases, 58.0% were white 42.0% were non-white; and among controls, 26.8% were white and 73.2% were non-white (P<0.01). Among cases, 38.0% were diagnosed with depression diagnosis and 62.0% were not. Among the controls, 17.1% were diagnosed with depression and 82.9% were not diagnosed with depression (P=0.03). Thirty six percent of cases had depressive symptoms > 9 as measured by the BDI and 64.0% has depressive symptoms < 9. Among the controls, 17.1% had depressive symptoms greater than 9 and 82.93% had depressive symptoms < 9 (P=0.04). Seventy-nine percent of cases diagnosed with depression had documented anti-depressive or anti-anxiety medications in their medical charts, and 21% did not have documented anti-depressive or anti-anxiety medication.
Table 1.
Demographic characteristics of cases and controls
| Variable | DED Cases (n=50) Mean ± SD |
Controls (n=41) Mean ± SD |
P-value* |
|---|---|---|---|
| Age | 52.6 ± 16.23 | 50.0 ± 14.75 | 0.43 |
| Gender | |||
| Male | 16 (32.0%) | 16 (39.0%) | 0.49 |
| Female | 34 (68.0%) | 25 (61.0%) | |
| Race | |||
| White | 29 (58.0%) | 11 (26.8%) | <0.01 |
| Non-White | 21 (42.0%) | 30 (73.2%) | |
| Depression | |||
| Yes | 19 (38.0%) | 7 (17.1%) | 0.03 |
| No | 31 (62.0%) | 34 (82.9%) | |
| BDI Depressive symptoms | |||
| > 9 | 18 (36.0%) | 7 (17.1%) | 0.04 |
| < 9 | 32 (64.0%) | 34 (82.9%) | |
Chi-square test for categorical variables, Fisher's exact test p-value when cell size <50, and student’s t-test for continuous variables
DED and depressive symptoms scores
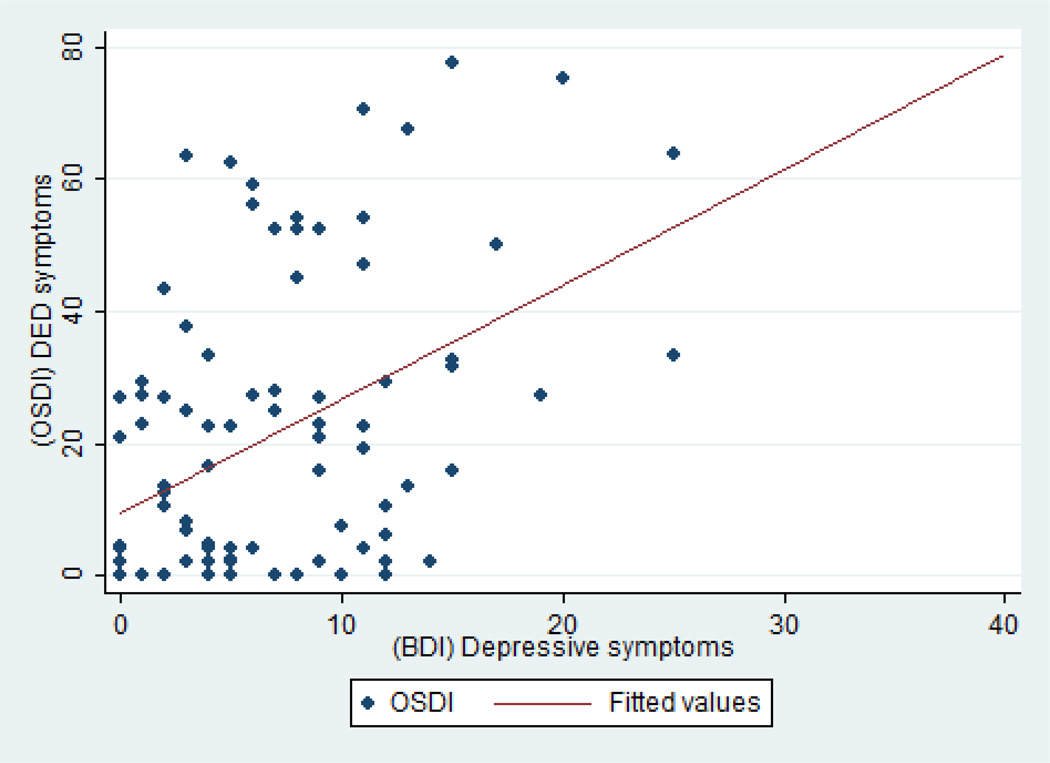
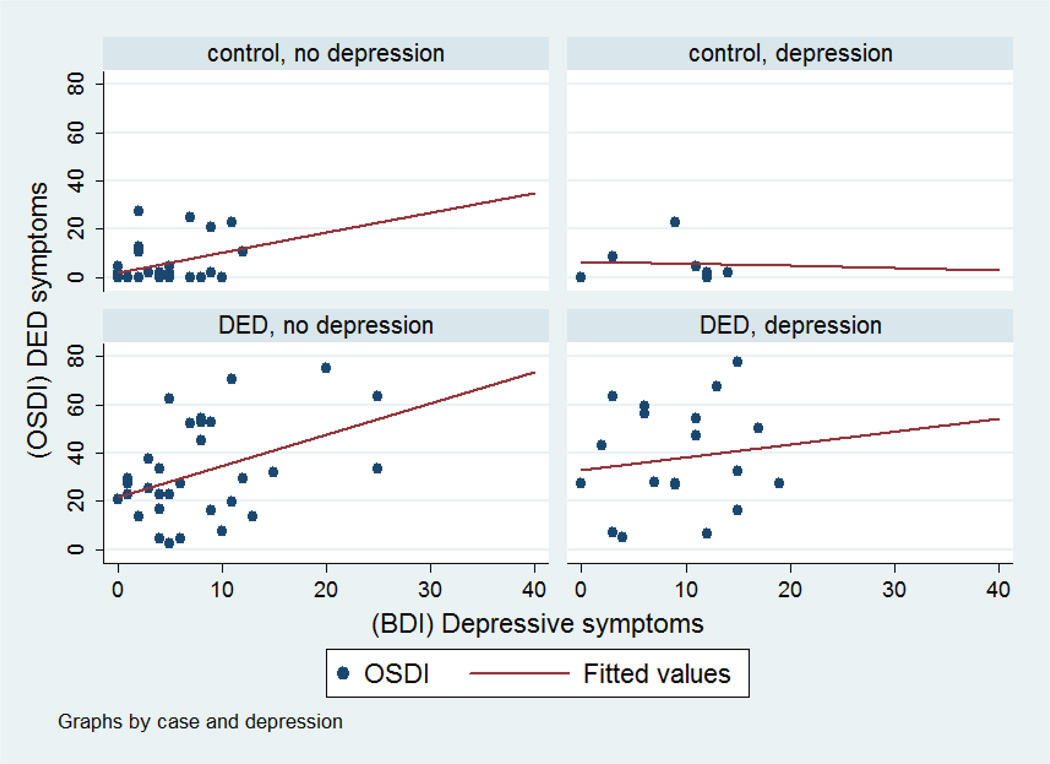
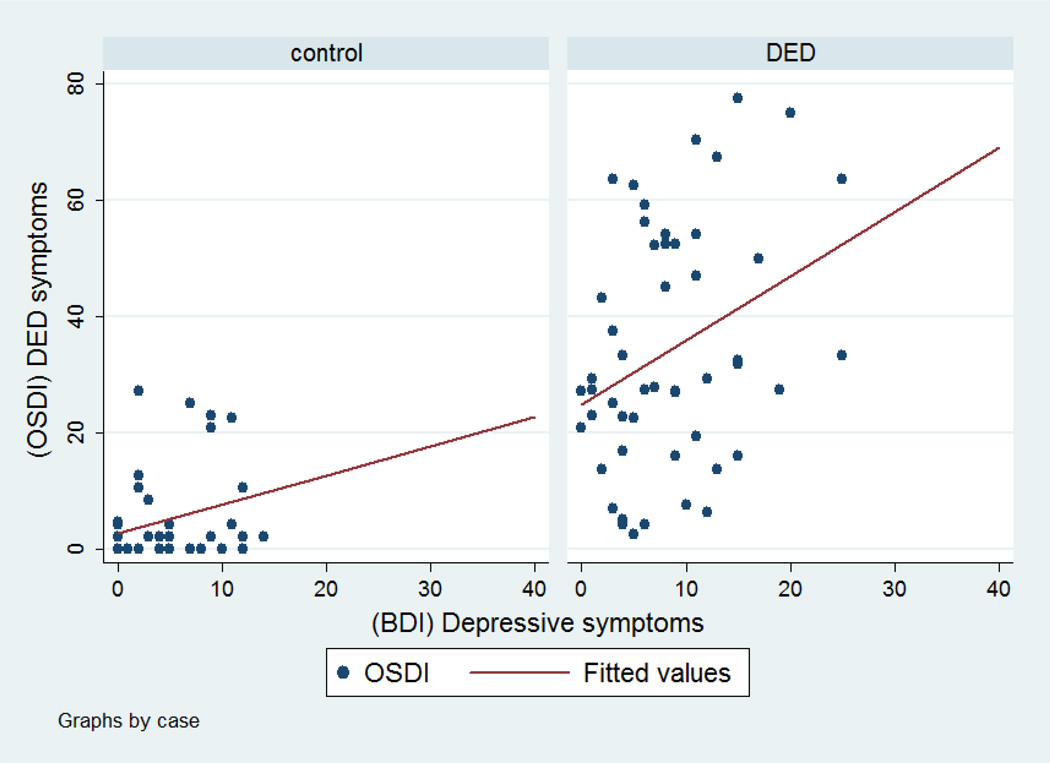
Figures 1–3 show scatter plots of depression symptoms against OSDI-DED symptoms for all subjects (Figure 1) for cases and controls (Figure 2) and further by depression status (Figure 3). Regression models revealed that the association is linear more than quadratic or cubic. The unadjusted regression coefficient for BDI depressive symptoms was 1.73 (95% CI 1.01–2.45) for all subjects. This means that with every one unit increase in BDI depression score, we expect a 1.73 unit increase in DED symptoms. After adjusting for age, gender, race, and psychiatric medication, the regression coefficient was 1.71 (95% CI 1.02, 2.40). Stratified by DED status, the regression coefficient for depressive symptoms was 1.11 (95% CI 0.17, 2.04) for cases and 0.50 (95% CI −0.05, 1.06) for controls. The adjusted regression coefficient between DED symptoms and depressive symptoms among DED cases was 1.22 (95% CI 0.27, 2.18).
Figure 1.
Scatter plots for the relationship between depressive symptoms as measured by the BDI questionnaire and DED symptoms as measured by the OSDI in the entire sample. The unadjusted regression coefficient for BDI depressive symptoms was 1.73 (95% CI 1.01–2.45). After adjusting for age, gender, race, and psychiatric medication, the regression coefficient was 1.71 (95% CI 1.02, 2.40).
Figure 3.
Scatter plots for the relationship between depressive symptoms as measured by the BDI questionnaire and DED symptoms as measured by the OSDI between DED cases and controls and clinical diagnosis of depression.
Figure 2.
Scatter plots for the relationship between depressive symptoms as measured by the BDI questionnaire and DED symptoms as measured by the OSDI between DED cases and controls. A simple linear regression between DED symptom scores and depressive symptom scores revealed a regression coefficient of 1.11 among DED cases and a regression coefficient of 0.50. The association was significant for DED cases (95% CI 0.17, 2.04) but not significant for controls (95% CI −0.05, 1.06). After controlling for age, gender, race, and psychiatric medication, the regression coefficient between DED symptoms and depressive symptoms among DED cases was 1.22 (95% CI 0.27, 2.18).
Table 2 lists the means for DED symptom scores and depression scores. The mean total OSDI score among DED cases was 34.18 compared to 4.78 among controls (P<0.001) and the mean total symptom burden questionnaire among DED cases was 16.95 versus 1.07 in controls (P<0.001) (Table 2). The mean BDI score was 8.44 ± 6.07 among DED cases and 4.32 ± 4.38 in controls (P<0.001). Among DED cases who have been diagnosed with depression, the mean OSDI scores and symptom burden scores were 37.94 and 19.38 compared to 31.88 and 15.46 among those without depression. Differences, however, were not statistically significant (P=0.32 and P=0.39). Mean BDI score among DED cases with depression was 9.32 compared to 7.9 among DED cases with no depression diagnosis. This result was also not statistically significant (P=0.43). Mean BDI score among controls with depression was 8.71 versus 3.41 among controls without depression (P=0.002). Logistic regression revealed an OR of 2.86 (95% CI 1.04, 7.87) for the association between DED and diagnosis of depression after controlling for age, gender, and race. Unadjusted logistic regression between DED status and BDI clinical category of > 9 revealed an OR of 2.73, 95% CI (1.0–7.4). Adjusted logistic regression revealed an OR of 2.79, 95% CI (0.96–8.12). This means that cases are 2.79 times more likely to report depressive symptoms of > 9 than controls, after adjusting for age, gender, race, and psychiatric medication.
Table 2.
DED and depression mean symptom scores
| Measure | DED Cases (n=50) Mean ± SD |
Controls (n=41) Mean ± SD |
P-value* |
|---|---|---|---|
| OSDI | |||
| Persistence | 6.14 ± 4.49 | 1.29 ± 2.87 | <0.001 |
| Activity | 4.22 ± 3.35 | 0.51 ± 1.19 | <0.001 |
| Environmental | 4.46 ± 3.36 | 0.83 ± 2.12 | <0.001 |
| Total Score | 34.18 ± 20.71 | 4.78 ± 1.22 | <0.001 |
| Symptom Burden | |||
| Persistence | 1.97 ± 1.10 | 0.22 ± 0.40 | <0.001 |
| Intensity | 6.98 ± 5.47 | 1.10 ± 1.91 | <0.001 |
| Activity | 1.45 ± 1.10 | 0.15 ± 0.37 | <0.001 |
| Affective | 1.07 ± 1.13 | 0.04 ± 0.15 | <0.001 |
| Total Score | 16.95 ± 15.41 | 1.07 ± 2.38 | <0.001 |
| BDI | 8.44 ± 6.07 | 4.32 ± 4.38 | <0.001 |
calculated using independent t-test
DISCUSSION
This case control study revealed three main findings: (i) A linear association between DED symptoms and depressive symptoms, which is more apparent among DED cases; (ii) DED cases with depression have higher DED symptom scores than DED cases without depression; and (iii) Clinical diagnosis of DED is associated with depression status and marginally associated with depressive symptoms, after controlling for age, gender, race, and psychiatric medication. These findings support the hypothesis regarding an association between DED and depressive symptoms. However, the mechanisms that underlie the association between depression, depressive symptoms, DED, and DED symptoms are unclear. We do not know whether DED and its symptoms are causing depression through chronic pain and the negative impact on daily activities, whether depression and its medication is causing DED, or is the relationship due to some other unmeasured factor causing both.
Several population-based studies have reported on the association between DED and depression3,4, 9. The strength of these studies lies in their representative samples and large sample sizes. However, the symptomatic assessment of both DED and depression was lacking. Additionally, these population studies were non-hypothesis based and relied on ICD-9 codes for ascertaining cases. Two population-based retrospective studies in the United States Veterans Affairs population in Miami reported high prevalence of depression in subjects with DED. In the first study, 17% of patients with a diagnosis of depression had DED as opposed to 10% without this diagnosis3. In the second study, 24% of patients with a diagnosis of depression had DED as opposed to 18% without this diagnosis4. The reported adjusted ORs for depression in both studies were comparable, 1.91 and 1.92 respectively3,4.
We reported an adjusted association between clinical diagnosis of DED and clinical diagnosis of depression of 2.86 in our study. This is comparable to other studies reporting on the association between depression and DED. In a population-based cross-sectional study of 657 Korean elders ≥ 65 years of age randomly selected from an official household registration database in Yongin Korea, Kim et al investigated the association between DED and depression and sought to evaluate the impact of co-morbid depression on the agreement of DED signs and symptoms (Kim KW 2011). DED symptoms were assessed using the 6-item dry eye questionnaire, and depressive symptoms were assessed using the Korean version of the Short Geriatric Depression Scale Depression. Depression was more prevalent in patients with DED (33.3% versus 18.1%). Adjusted analyses revealed depression as an independent risk factor for DED (OR 3.08; 95% CI 1.93–4.93). The authors of this study listed several limitations including the lack of assessment of medication, the cross-sectional nature of the study, and the severity of DED was assessed using the dry eye questionnaire. Subjective symptoms may be better quantified using tools including the visual analog scale and the ocular surface disease index score, especially when using symptom-based diagnostic criteria for DED6, 19, 21.
In another recent population based case-control study in Taiwan, Wang et al investigated the co-morbidities of DED22. They used a nationwide subset database released by the Taiwan National Health Research Institute (NHRI) in 2006. The program to create the database covered 22 million enrollees, representing over 98% of the island’s population. The NHRI used a systematic, random sampling method to extract 5% of the enrollees (n=1,073,891). The DED cases consisted of 12,007 patients (after excluding patients under 18 years of age) who sought ambulatory care with a principal diagnosis of DED and 36,021 randomly selected controls. The prevalence of psychiatrically diagnosed depression was higher in patients with DED (7.20% versus 3.55%) and the adjusted measure of association (OR) reported was 2.11 (95% CI 1.93–2.31)22. ICD-9 CM codes were used for the diagnosis of DED and depression but the symptoms were not assessed. Additionally, depression was included among other comorbidities such as heart disease, systemic lupus, asthma, pulmonary circulation disorders, diabetes, liver diseases, and solid tumors and metastasis. A more recent retrospective case control study performed at the University of North Carolina outpatient clinics, reported an adjusted OR for DED and anxiety of 2.8 and an adjusted OR for DED and depression of 2.9. Outcome and exposure variables were also assessed using ICD-9 diagnosis codes9.
In this study, we recruited patients from our DED clinic and assessed the symptoms of both DED and depression using validated questionnaires. Additionally, Depression status was determined as a composite variable through chart review as "ever having depression" through medical and psychological history and/or through current prescribed medications or any history of prescribed medication. Seventy seven percent of cases diagnosed with depression were actively on anti-depressive or anti-psychiatric medications at the time of questionnaire administration, and 23% either had some history of depression or were prescribed anti-depression medication. This allowed us to control for psychiatric medication in our analysis between depressive symptoms and DED symptoms. Due to the possibility of under-recording of medication use in charts, we also analyzed the data according to depression status and the interpretation of our results was the same. We also showed that depressive symptom scores among DED patients do not vary between depressed and not-depressed patients. This means that patients with DED exhibit depressive symptoms regardless of depression status.
There are several limitations to our study. First the sample size is not large to make definite conclusions and our results cannot be generalized to the general population. Additionally, our medication assessment for depression is not complete; while current use was used to determine depression status, we did not determine the frequency of medication, dosage, and the various types of medication. These medications may have different effects on symptoms at the time of questionnaire administration. This may have impacted our results, where patient symptoms may have varied with different medications. Similarly, this applies to our assessment of DED symptoms without assessing aggressiveness of treatment. Additionally, we did not control for other co-morbidities that may be related to both DED and depression. This study also lacked information on the socio-economic and insurance statuses of the patients.
Despite our limitations, this study provides further evidence regarding the association between DED and depression and their symptoms. Prospective studies that additionally consider a direct comparison of group of DED with depression to group of controls with depression, and account for medication usage, co-morbodities, and socioeconomic status are needed to understand the mechanisms underlying the association between symptoms of depression and symptoms of DED.
Footnotes
Conflict of Interest : None declared
REFERENCES
- 1.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 2.Hallak JA, Jassim S, Khanolkar V, et al. Symptom burden of patients with dry eye disease: a four domain analysis. PLoS One. 2013;8:e82805. doi: 10.1371/journal.pone.0082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152:377–384. doi: 10.1016/j.ajo.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012;154:340–346. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Wen W, Wu Y, Chen Y, et al. Dry eye disease in patients with depressive and anxiety disorders in Shanghai. Cornea. 2012;31:686–692. doi: 10.1097/ICO.0b013e3182261590. [DOI] [PubMed] [Google Scholar]
- 6.Kim KW, Han SB, Han ER, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52:7954–7958. doi: 10.1167/iovs.11-8050. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Gong L, Sun X, et al. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36:1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 8.Labbé A, Wang YX, Jie Y, et al. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97:1399–1403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 9.van der Vaart R, Weaver MA, Lefebvre C, et al. The Association Between Dry Eye Disease and Depression and Anxiety in a Large Population-Based Study. Am J Ophthalmol. 2015;159:470–474. doi: 10.1016/j.ajo.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anttila SS, Knuuttila ML, Sakki TK. Depressive symptoms as an underlying factor of the sensation of dry mouth. Psychosom Med. 1998;60:215–218. doi: 10.1097/00006842-199803000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Bergdahl M, Bergdahl J, Johansson I. Depressive symptoms in individuals with idiopathic subjective dry mouth. J Oral Pathol Med. 1997;26:448–450. doi: 10.1111/j.1600-0714.1997.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 12.Vriezekolk JE, Geenen R, Hartkamp A, et al. Psychological and somatic predictors of perceived and measured ocular dryness of patients with primary Sjögren's syndrome. J Rheumatol. 2005;32:2351–2355. [PubMed] [Google Scholar]
- 13.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [No authors listed] (2007) [DOI] [PubMed] [Google Scholar]
- 14.Li M, Gong L, Chapin WJ, et al. Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5722–5727. doi: 10.1167/iovs.11-9094. [DOI] [PubMed] [Google Scholar]
- 15.Thieme K, Turk DC, Gracely RH, et al. The relationship among psychological and psychophysiological characteristics of fibromyalgia patients. J Pain. 2015;16:186–196. doi: 10.1016/j.jpain.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 20.Dougherty BE, Nichols JJ, Nichols KK. Rasch analysis of the Ocular Surface Disease Index (OSDI) Invest Ophthalmol Vis Sci. 2011;52:8630–8635. doi: 10.1167/iovs.11-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitale S, Goodman LA, Reed GF, et al. Comparison of the NEI-VFQ and OSDI questionnaires in patients with Sjögren's syndrome-related dry eye. Health Qual Life Outcomes. 2004;2:44. doi: 10.1186/1477-7525-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TJ, Wang IJ, Hu CC, et al. Comorbidities of dry eye disease: a nationwide population-based study. Acta Ophthalmol. 2012;90:663–668. doi: 10.1111/j.1755-3768.2010.01993.x. [DOI] [PubMed] [Google Scholar]