Abstract
Rationale
Transplantation, the most effective therapy for end-stage organ failure, is markedly limited by early-onset cardiovascular disease (CVD) and premature death of the host. The mechanistic basis of this increased CVD is not fully explained by known risk factors.
Objective
To investigate the role of alloimmune responses in promoting CVD of organ transplant recipients.
Methods and Results
We established an animal model of graft-exacerbated host CVD by combining murine models of atherosclerosis (apolipoprotein E-deficient recipients on standard diet) and of intra-abdominal graft rejection (heterotopic cardiac transplantation without immunosuppression). CVD was absent in normolipidemic hosts receiving allogeneic grafts and varied in severity among hyperlipidemic grafted hosts according to recipient-donor genetic disparities, most strikingly across an isolated major histocompatibility complex class II antigen barrier. Host disease manifested as increased atherosclerosis of the aorta that also involved the native coronary arteries and new findings of decreased cardiac contractility, ventricular dilatation, and diminished aortic compliance. Exacerbated CVD was accompanied by greater levels of circulating cytokines, especially interferon-γ and other Th1-type cytokines, and showed both systemic and intra-lesional activation of leukocytes, particularly T helper cells. Serologic neutralization of interferon-γ after allotransplantation prevented graft-related atherosclerosis, cardiomyopathy, and aortic stiffening in the host.
Conclusions
Our study reveals that sustained activation of the immune system due to chronic allorecognition exacerbates the atherogenic diathesis of hyperlipidemia and results in de novo cardiovascular dysfunction in organ transplant recipients.
Keywords: Transplantation, atherosclerosis, allograft rejection, T cell, interferon-γ, lymphocyte
INTRODUCTION
Transplantation is the most effective, and in certain cases the only, therapy for patients with end-stage organ failure. Medical advances have substantially improved outcomes, although this is mostly attributed to dramatically increased patient and graft survival in the first year to over 80-90% with minimal changes in the longer-term attrition in patient or graft survival of 4-5% per year1. While graft loss often translates into patient demise after liver or heart transplantation, artificial means of support and re-transplantation is more applicable to salvage recipients of failed kidney grafts allowing for greater separation of patient survival from graft function. In a notable study with complete follow-up of kidney transplant recipients irrespective of graft function, death with a functioning graft, mostly from cardiovascular disease (CVD), was the most common cause of graft loss2. Other single institution and national registry studies have confirmed a leading role for CVD in renal transplant recipient deaths3,4. CVD deaths occur 3-5 times more frequently in renal transplant recipients than in the general population which translates to premature mortality rates of 10-20 years5. CVD is also a leading cause of non-graft related death in liver transplant recipients6, and extracardiac vascular disease is a significant problem in heart transplant recipients7. The substantial impact of CVD in transplant recipients is well-recognized, however current therapy with conventional medications, such as statins, have had limited success8.
It is widely believed that the increased incidence of CVD in renal transplant recipients results from a preponderance of traditional risk factors, such as hypertension, hyperlipidemia, and diabetes due to their common occurrence in patients with chronic kidney diseases before transplantation as well as the untoward effects of immunosuppressive drugs after transplantation. Whilst partially true, the Framingham Risk Score, based on these traditional risk factors and validated to predict future cardiovascular events in the general population, consistently and substantially underpredicts CVD morbidity and mortality in the kidney transplant population9-13. The restricted predictive value of traditional risk factors for CVD in transplant recipients is interpreted to indicate the effect of additional, unidentified disease precipitants14. Instead of “Framingham” criteria, the strongest risk factors for CVD events in renal transplant patients are pre-existing atherosclerosis and indices of poor graft function13-16. In turn, the most common cause of graft dysfunction is chronic rejection17. Further evidence for immunological causation is that circulating markers of inflammation, such as IL-6, C-reactive protein, and erythropoietin, are independently associated with CVD in renal transplant recipients18,19.
Premature CVD in renal transplant recipients has diverse clinical presentations. Although myocardial infarctions are common complications, an even greater increase in incidence has been noted in heart failure and sudden (presumed arrhythmic) cardiac deaths8,20-22. This skewed presentation of CVD is another difference, besides that of risk factors, between renal transplant recipients and the general population that points to novel pathogenetic mechanisms14. Accelerated heart failure independent of atherosclerosis may be due to ventricular hypertrophy and aortic stiffening that cause diastolic dysfunction and alter ventricular-aortic coupling during systole23. Indeed, markers of ventricular strain and aortic stiffening were univariate determinants of cardiac deaths, but not of non-fatal myocardial infarctions, in kidney transplant recipients10. In another study, graft dysfunction and circulating C-reactive protein levels, but not traditional CVD risk factors, independently correlated with increased arterial stiffening in kidney transplant recipients compared to controls24. Thus, particular manifestations of graft-related CVD may be precipitated by different risk factors and common pathogenetic mechanisms cannot be presumed.
An improved understanding of CVD pathogenesis in the setting of organ transplantation is required for progress in preventing and treating the disease. In addition to the valuable insight gained from clinical outcomes studies, basic science investigations in model organisms are necessary to define unrecognized causes of CVD in the transplant recipient, to dissect their mechanisms, and to identify therapeutic targets. Using a novel experimental model of graft-related host CVD, we find that intra-abdominal graft rejection exacerbates atherosclerosis and induces de novo cardiovascular dysfunction in hyperlipidemic mouse recipients by IFN-γ-mediated immune and inflammatory responses.
METHODS
Mice
C57BL/6J, BALB/cJ, B61-H2-Ab1bm12/KhEgJ (bm12), B6.C-H2-Kbm1/ByJ (bm1), and B6.129P2-Apoetm1Unc/J (ApoE−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were housed according to institutional guidelines and were fed a standard chow diet. Male mice were exclusively analyzed, except for female ApoE−/− recipients of minor-mismatched grafts and their controls. Surgical procedures were performed with 6-12 wk-old donor mice and 30 wk-old recipient mice. Certain recipients were treated with neutralizing monoclonal antibody to IFN-γ (clone R4-6A2, BioXCell, West Lebanon, NH) or an isotype-matched, irrelevant rat IgG1 antibody (clone HRPN, BioXCell) at a loading dose of 250 μg, sc, 1 d prior followed by 125 μg, sc, 3× per wk, from 0-12 wk after transplantation25. The animals were euthanized at 42 wk of age for analysis.
Surgical procedures
Intra-abdominal heterotopic cardiac transplantation was performed by end-to-side anastomosis of the donor ascending aorta and pulmonary trunk to the recipient infra-renal aorta and inferior vena cava, respectively. The animals were observed daily after surgery; severely ill recipients (all of which had rejected their grafts, except for 1 minor-mismatched recipient) were euthanized and adjudicated as deaths. Graft rejection was determined by abdominal palpation of the donor heartbeat. Alternatively, full thickness donor skin grafts were engrafted onto the back of recipient mice and covered with gauze and a securing bandage for 7 d. The animals were observed daily after surgery. Graft rejection was defined as necrosis >80% surface area. All animal studies were approved by Yale University's Institutional Animal Care and Use Committee.
Analytical techniques
The experimental methods are described in the online supplement.
Statistical analysis
Data represent mean±SEM. Unpaired Student's t-test was used for comparisons between 2 groups, one-way analysis of variance was used for comparisons between >2 groups, and the logrank test was used to compare survival curves. Differences with two-tailed P values <0.05 were considered to indicate statistical significance. Statistical analyses were performed using Prism 4.0 (GraphPad Software, La Jolla, CA).
RESULTS
Experimental model of graft-related CVD
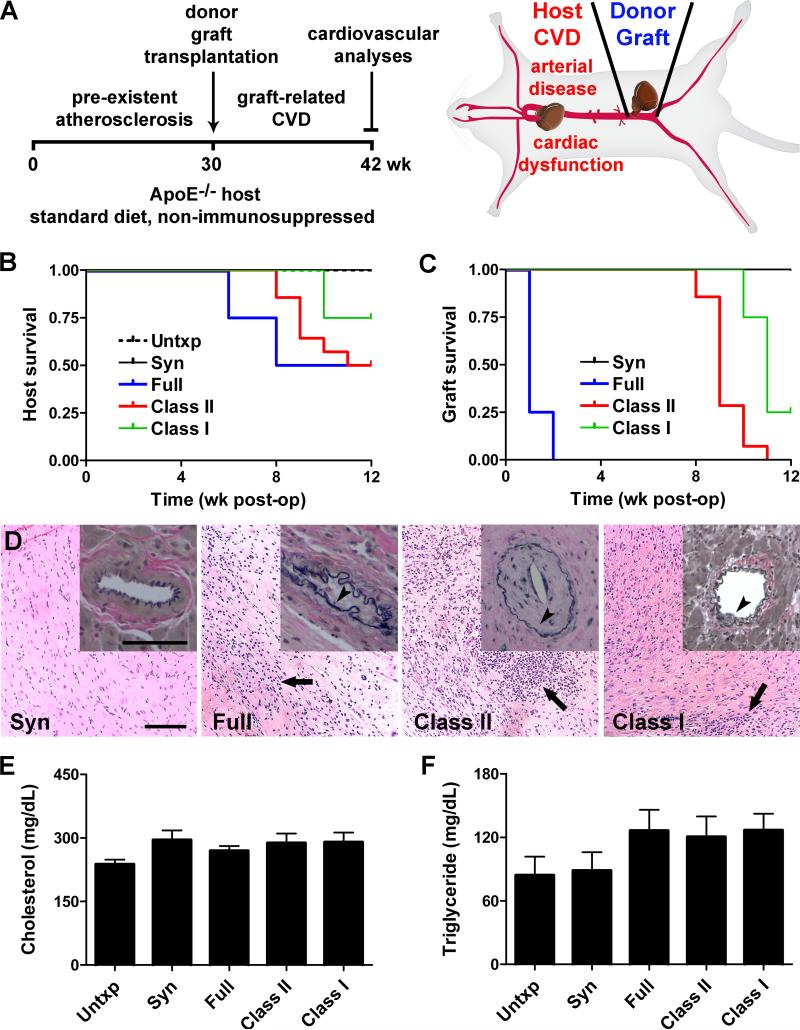
To study the pathogenesis of CVD in transplant recipients, we combined experimental models of atherosclerosis and graft rejection26,27. We used apolipoprotein E-deficient (ApoE−/−) mice at 30 wk of age as hosts to simulate the clinical risk factor of pre-existent atherosclerosis15-18, regular chow to avoid anti-Th1 effects of high-fat diets28, heterotopic abdominal cardiac grafts as the transplanted organ for technical convenience and ease of monitoring function, no immunosuppression to permit allograft rejection and exclude confounding pharmacologic effects, and 12 wk surveillance after transplantation to enable alloimmune responses to modulate host CVD (Figure 1A). Although major histocompatibility complex (MHC) and minor histocompatibility antigens expressed by the allograft may elicit immune rejection, it is not known which, if any, of these responses may elicit host CVD. We therefore screened several donor-recipient genetic disparities; controls included untransplanted and syngeneic-grafted animals. All the operations were technical successes (defined as host survival >3 d with beating cardiac grafts), however several of the recipients died unexpectedly or were euthanized due to poor health after their allografts had rejected between 6-12 wk post-op (Figure 1B). In contrast, there were no deaths of untransplanted or syngeneic-grafted ApoE−/− mice. The deaths of ApoE−/− recipients with allogeneic grafts was unexpected as normolipidemic recipients with similar antigen-mismatched grafts are known to survive long-term27. Autopsies did not reveal direct graft causes of death, e.g. hemorrhage or thrombosis. As expected, ApoE−/− male recipients acutely rejected full haplotype-mismatched hearts, chronically rejected either single MHC class II or class I antigen-mismatched hearts, and did not reject syngeneic hearts (Figure 1C,D). Additionally, ApoE−/− female recipients of H-Y minor histocompatibility antigen-mismatched male hearts exhibited less graft attrition with fewer histological signs of rejection than for isolated MHC antigen differences (Online Figure I). The recipient groups are, henceforth, abbreviated as full-, class II-, class I-, or minor-mismatched. Cholesterol and triglyceride serum levels did not differ between the various donor-recipient combinations (Figure 1E,F). Thus, the transplanted hosts with similar degrees of hyperlipidemia were suitable to examine for graft-related differences in CVD.
Figure 1. Experimental model of graft-related CVD.
(A) ApoE−/− hosts fed regular chow received a heterotopic graft at 30 wk of age without immunosuppression and recipient cardiovascular function and disease was assessed at 12 wk post-op. (B) Survival of C57BL/6 ApoE−/− male mice after no transplantation (Untxp) or after transplantation with syngeneic (C57BL/6, H2b), full-mismatched (BALB/c, H2d), class II-mismatched (bm12, H2-Abm12), and class I-mismatched (bm1, H2-Kbm1) heterotopic cardiac grafts; P<0.05. (C) Graft survival as determined by daily abdominal palpation for cardiac pulsation; P<0.001. (D) Representative photomicrographs of the donor grafts at 12 wk post-op showing cardiomyocyte destruction with cellular infiltrates (arrows, H&E stain) and coronary artery neointima formation (insets, arrowheads, EVG stain); bars=100 μm. (E) Total cholesterol and (F) triglyceride serum levels of hosts at 8-12 wk post-op, n=4, P=0.23 and P=0.29, respectively.
Graft rejection is associated with increased atherosclerosis of the host
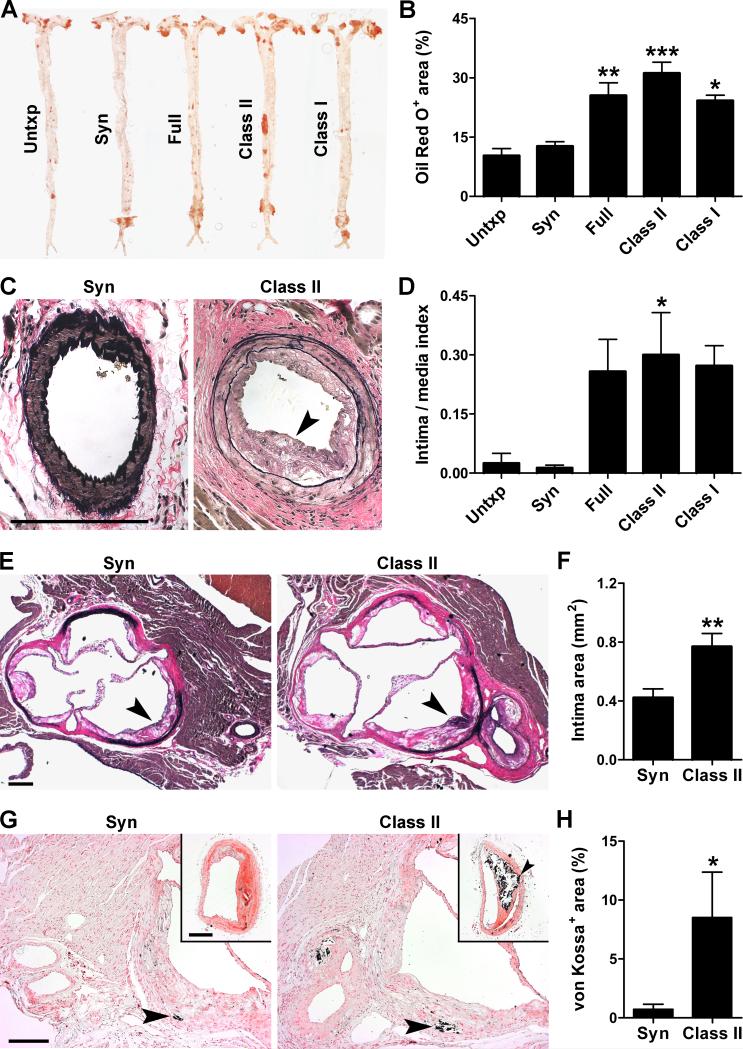
Animals were euthanized at 12 wk post-op (or earlier if distressed) to assess the extent of host atherosclerotic disease. Aortic lipid deposition as measured by Oil Red O staining was 2-3 fold greater after allogeneic than syngeneic grafting or no transplantation (Figure 2A,B). Strikingly, the atherosclerotic lesions extended into the proximal segments of native coronary arteries, most consistently in recipients of class II-mismatched grafts (Figure 2C,D). In an additional group of animals in which the entire aorta was not processed for en face lipid staining, larger neointimal lesions were also found in cross-sections of the aortic root in recipients of class II-mismatched vs. syngeneic grafts (Figure 2E,F). The atherosclerotic plaques of allogeneic recipients were characterized by greater mineralization on von Kossa staining, a feature of advanced disease (Figure 2G,H). Increased calcification was confirmed by Alizarin red staining (not shown). Recipients of minor-mismatched grafts exhibited a lesser 1.5-2 fold increase in aortic atherosclerosis and no coronary artery lesions (Online Figure I). In contrast, allograft rejection in the absence of hyperlipidemia was not sufficient to induce atherosclerotic plaques in the aorta (nonspecific staining at the anastomotic site notwithstanding) or coronary arteries of wild-type hosts (Online Figure II). Furthermore, class II-mismatched skin grafts, which underwent more rapid tissue necrosis within 2 wk, only minimally increased aortic lipid deposition without coronary artery lesions in ApoE−/− recipients (Online Figure III). These data suggest that both host (pre-existent vascular disease) and graft (persistent alloimmune responses) factors are required to produce the accelerated atherosclerosis phenotype of our model.
Figure 2. Graft rejection exacerbates pre-existing atherosclerosis of the host.
ApoE−/− mice on standard diet were either not grafted or received heterotopic cardiac grafts of varying genetic disparities at 30 wk of age and atherosclerotic disease of the recipient was analyzed after 8-12 wk. (A) Representative Oil Red O stains of host aortas and (B) expressed as % of total area. (C) Representative EVG stains of host coronary arteries showing neointima (arrowhead) and (D) expressed as intima to media area index. (E) Representative EVG stains of host aortic roots showing neointima (arrowheads) and (F) measured as intima area. (G) Representative von Kossa stains of host aortic roots and brachiocephalic arteries (insets) showing mineralization (arrowheads) and (H) expressed as % of plaque area. Bars=200 μm. Data was pooled from 3 independent experiments, n=3-4 Untxp, Full, and Class I, n=7-12 Syn and Class II, *P<0.05, **P<0.01, ***P<0.001 vs. Untxp or Syn (there were no significant differences between the Untxp and Syn control groups or between the Full, Class II, and Class I allogeneic groups).
Graft rejection induces de novo cardiovascular dysfunction of the host
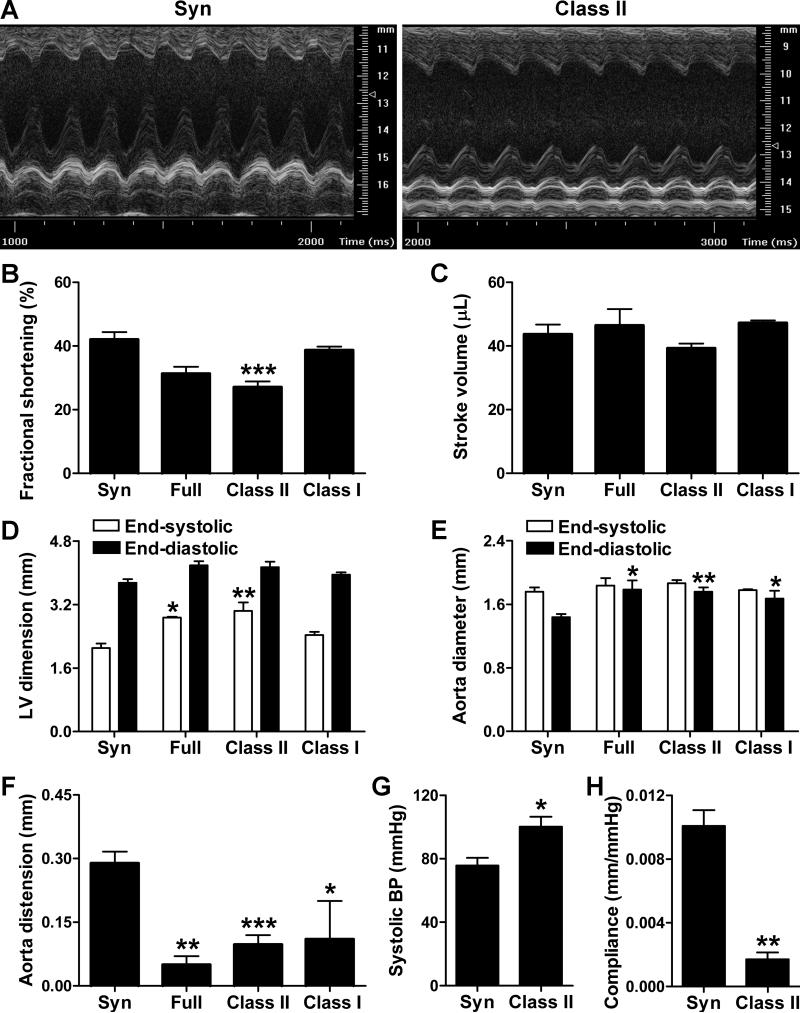
Prior to euthanasia, cardiac and aortic function of anesthetized hosts was assessed by echocardiography (Figure 3A). Indices of left ventricular systolic function, such as fractional shortening, were significantly decreased in recipients of class II-mismatched vs. syngeneic grafts, although stroke volume was preserved (Figure 3B,C). Cardiac output was maintained (not shown) at the expense of ventricular dilatation, particularly at end-systole (Figure 3D). Aortic size was also increased in recipients of allogeneic vs. syngeneic grafts, particularly at end-diastole (Figure 3E). These diameter changes translated as diminished aortic distension and recoil during the cardiac cycle (Figure 3F). Alterations in cardiovascular function were confirmed by invasive monitoring with a high-fidelity pressure sensor in a subgroup of animals. Compared to hosts of syngeneic grafts, recipients of class II disparate grafts had increased systolic blood pressure (Figure 3G), but not diastolic blood pressure (69.9±5.6 vs. 48.5±6.6 mmHg, P=0.07), pulse pressure (30.3±2.6 vs. 27.2±2.0 mmHg, P=0.38), or heart rate (327±10 vs. 348±39 min−1, P=0.64). Peak rates of left ventricular pressure change were similar under basal conditions, but the allogeneic hosts demonstrated blunted inotropic and lusitropic responses to dobutamine infusion at 4 μg/kg/min (17.7±8.5% vs. 55.8±6.7% increase in dP/dtmax, P<0.05 and 6.4±10.7% vs. 66.5±12.9% increase in dP/dtmin, P<0.05). Calculation of arterial compliance based on distension and blood pressure measurements confirmed increased aortic stiffness in ApoE−/− recipients of class II-mismatched grafts (Figure 3H). Cardiovascular dysfunction was not apparent in ApoE−/− recipients of minor-mismatched cardiac grafts or class II-mismatched skin grafts and in normolipidemic WT recipients of class II-mismatched cardiac grafts, except for diminished responses to dobutamine infusion in the latter group (Online Figure I-III). Thus, cardiac and thoracic aorta dysfunction of the hyperlipidemic host correlated with chronic allograft rejection in a remote body compartment.
Figure 3. Graft rejection induces de novo cardiovascular dysfunction of the host.
(A) Representative M mode echocardiography of the native heart in ApoE−/− recipients of syngeneic and class II-mismatched grafts at 12 wk post-op. Echocardiographically-determined (B) fractional shortening, (C) stroke volume, (D) left ventricular (LV) internal dimensions, (E) ascending aorta diameters, and (F) ascending aorta distension of the host after syngeneic, full-, class II-, and class I-mismatched grafts; data was pooled from 3 independent experiments, n=2 Full and Class I, n=8-11 Syn and Class II, *P<0.05, **P<0.01, ***P<0.001 vs. Syn. Invasively determined (G) central systolic blood pressure and (H) aortic compliance (i.e. distension/pulse pressure) in recipients of syngeneic and class II-mismatched grafts; data from a single experiment, n=3, *P<0.05, **P<0.01 vs. Syn.
Graft-exacerbated CVD is associated with systemic and cardiovascular inflammation
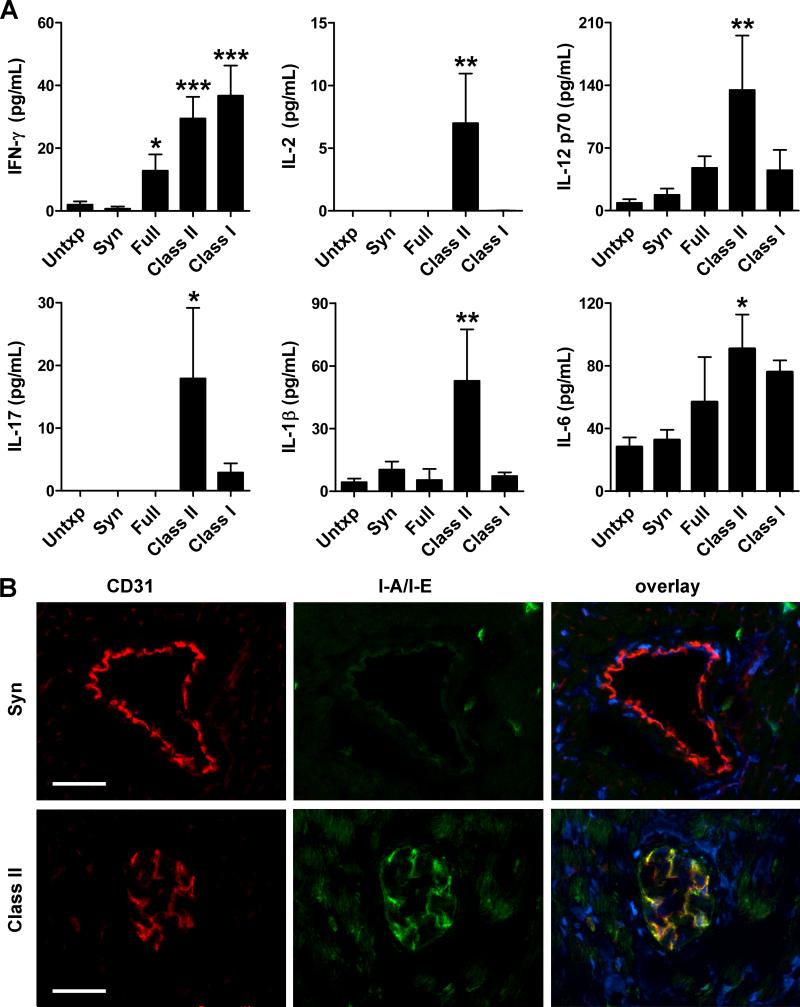
To determine how donor graft rejection within the abdomen may modulate host CVD in the thorax, we examined for evidence of circulating proinflammatory cytokines using multiplex bead-based immunoassays. Elevated plasma levels of the prototypic Th1 cytokine, interferon (IFN)-γ and the prototypic Th17 cytokine, interleukin (IL)-17 were found in recipients of class II-mismatched grafts compared to syngeneic-grafted or untransplanted ApoE−/− hosts at 12 wk post-op (Figure 4A). This was associated with increased plasma levels of the IFN-γ inducers, IL-2 and IL-12, as well as the IL-17 inducers, IL-1β and IL-6 (Figure 4A). IL-12 p40 (a component of both IL-12 and the IL-17 inducer, IL-23) was also upregulated, but there were undetectable levels or no significant changes in multiple other cytokines assayed, including Th2, Th3, and Th9 lymphokines and macrophage growth factors (Online Figure IV). IFN-γ was also significantly elevated in full- and class I-mismatched recipients and trended to significant elevation in minor-mismatched recipients, but other changes of Th1- and Th17-related cytokines did not show consistent elevation across all allograft combinations (Figure 4A, Online Figure I). Since class II-mismatched recipients showed the greatest induction in circulating cytokines, we further examined the cardiovascular system of these hosts for inflammatory sequelae. A biological response to circulating cytokines could be inferred from increased expression of IFN-γ-inducible MHC class II antigens by host coronary artery endothelial cells (Figure 4B). Although circulating levels of immunoglobulin were unchanged, antibody deposition was noted in the host myocardial vasculature and we documented that the expression of autoantigens by cultured vascular cells was upregulated by IFN-γ (Online Figure V). Finally, greater infiltrates of mononuclear leukocytes, particularly of T cells, were noted in host atherosclerotic aortic lesions in situ by immunofluorescence analysis, whereas sparse infiltrates of granulocytes were similar to controls (Online Figure VI).
Figure 4. Systemic and cardiovascular inflammation in recipients of rejecting allografts.
(A) Plasma levels of IFN-γ, IL-2, IL-12 p70, IL-17, IL-1β, and IL-6 in ApoE−/− recipients of syngeneic or full-, class II-, and class I-mismatched grafts at 12 wk post-op; n=3-8, *P<0.05, **P<0.01, ***P<0.001 vs. Untxp and Syn. (B) Representative images of immunofluorescence analysis of native coronary arteries in recipients of syngeneic (upper panels) and class II-mismatched (lower panels) grafts for the endothelial marker, CD31 (red color), for IFN-γ-inducible I-A/I-E MHC class II antigens (green color), and overlayed together with DAPI nuclear staining (blue color), bars=50 μm. Host coronary arteries in allografted animals have partially occluded lumens and increased endothelial expression of I-A/I-E antigens.
Graft-exacerbated CVD is associated with systemic and intra-lesional T cell activation
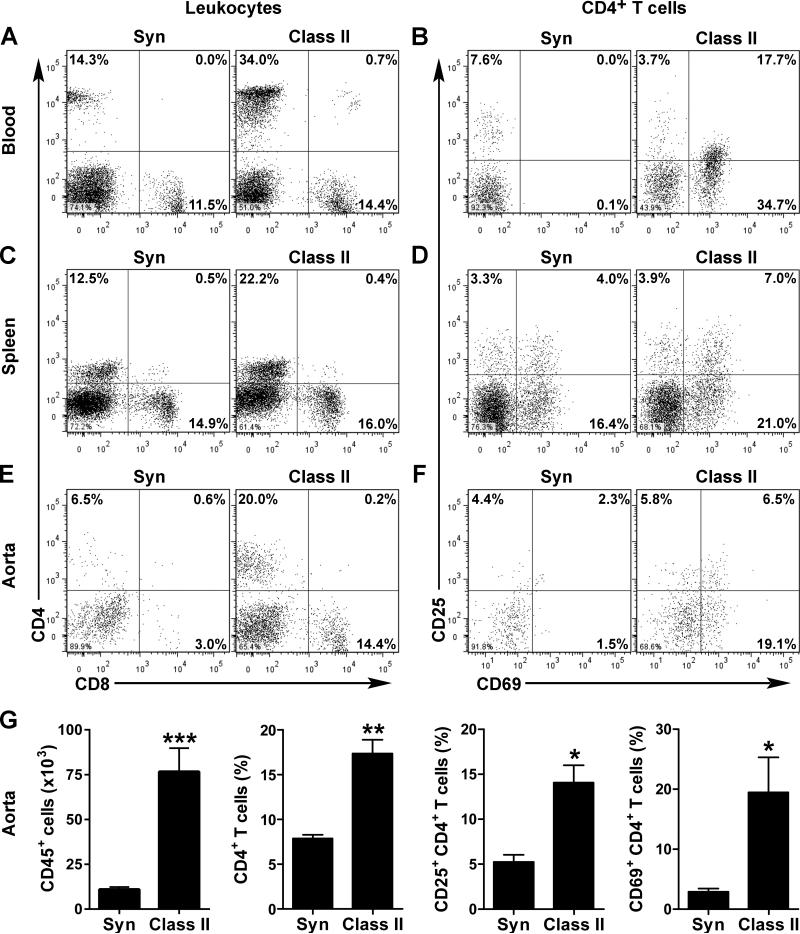
We further characterized the phenotype of leukocytes isolated from ApoE−/− mice that received class II-mismatched vs. syngeneic grafts by flow cytometry. At 12 wk after allotransplantation, the frequency of circulating lymphocytes, in particular CD4+ T helper cells, was increased (Figure 5A). Furthermore, these cells displayed a greater expression of the activation markers, CD25 and CD69 (Figure 5B). Interestingly, the CD25high/CD4+ subpopulation, typically regulatory T cells, did not increase in frequency or acquire CD69 expression. The activation of circulating CD4+ T cells was far less apparent at 4 and 8 wk post-op (Online Figure VII). The number of circulating activated CD4+ T cells was only modestly increased in normolipidemic hosts of cardiac allografts and unchanged in hyperlipidemic hosts of skin allografts (Online Figure II,III). Similar to the findings in the circulation, the proportion of activated CD4+ T cells was higher in the spleens of ApoE−/− recipients bearing class II-mismatched grafts (Figure 5C,D). Notably, there were also more CD4+ T cells with greater expression of CD25 and CD69 infiltrating the native aorta of these animals (Figure 5E,F). The differences in activated T helper cells was even more significant considering 7-fold more CD45+ leukocytes were isolated from the aortas of allogeneic than syngeneic recipients (Figure 5G). There was also an increased frequency and total number of activated (CD25+/CD69+) CD8+ cytotoxic T cells, inflammatory (CCR2+/Ly6Chigh) CD11b+ monocytes, and proatherogenic (CD5−/CD43−) B220+ B-2 cells (Online Figure VIII,IX), however these were of lesser magnitude than the changes in CD4+ T cells. On the other hand, the frequency of patrolling (CCR2−/Ly6Clow) monocytes, atheroprotective (CD5+/CD43+) B-1a cells, and Ly6G+ neutrophils were lower in allografted animals (Online Figure VIII,IX). Together, the elevated levels of circulating cytokines and increased number of activated leukocytes demonstrated greater activation of the immune system in hyperlipidemic recipients of rejecting allografts that correlated with the occurrence of exacerbated host CVD.
Figure 5. Systemic and intra-lesional T cell activation in recipients of rejecting allografts.
(A) Flow cytometric analysis for CD4 and CD8 expression by circulating mononuclear leukocytes in ApoE−/− recipients of syngeneic and class II-mismatched grafts at 12 wk post-op. (B) Circulating CD4+ T cells were further analyzed for expression of the activation markers, CD25 and CD69. Similar analyses in (C,D) splenocytes and (E,F) host aorta-infiltrating leukocytes. Cells for each analysis were pooled from 2-3 animals. (G) Additionally, data from 3-4 independent experiments were pooled to calculate the mean number of CD45+ leukocytes and the frequency of CD4+, CD25+/CD4+, and CD69+/CD4+ T cells infiltrating the host aorta; n=3, *P<0.05, **P<0.01, ***P<0.001 vs. Syn.
IFN-γ promotes graft-exacerbated CVD in the transplant recipient
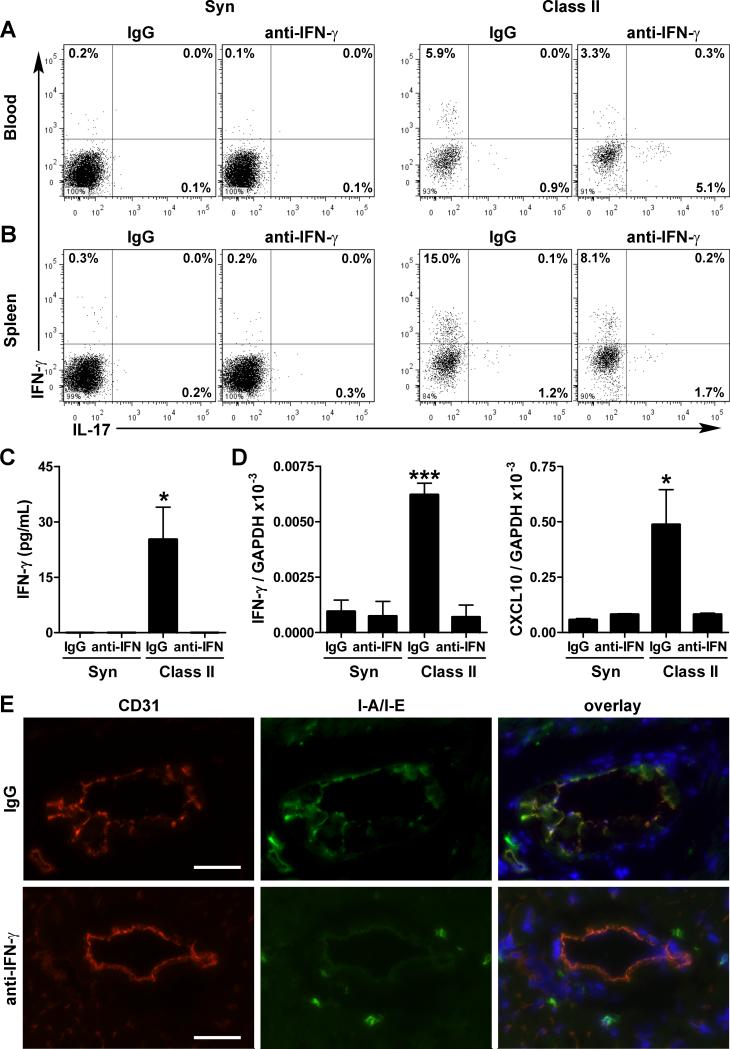
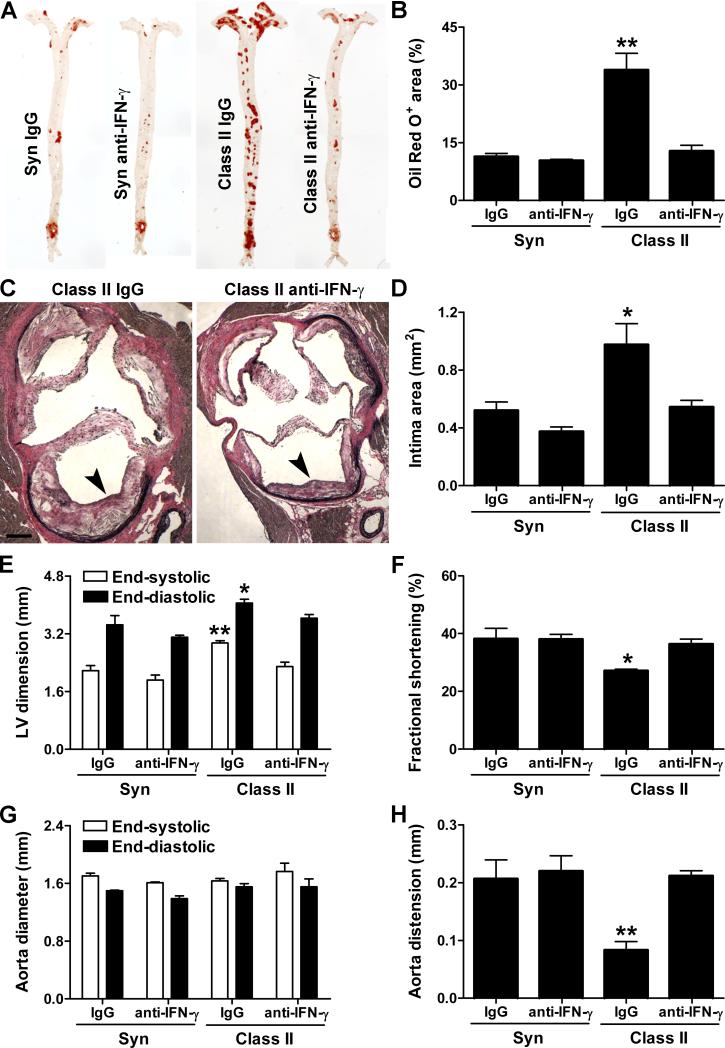
Finally, we asked if IFN-γ could account for the increased severity of CVD in ApoE−/− mice receiving an allograft as, in addition to higher plasma levels, synthesis of this key pro-arteriosclerotic factor by CD4+ T cells in the circulation and secondary lymphoid tissues was disproportionately increased (30-50-fold more Th1 cells compared to 6-fold more Th17 cells) in recipients of class II-mismatched vs. syngeneic grafts (Figure 6A,B). There was also increased production of IFN-γ by leukocytes other than CD4+ T cells (but of lesser magnitude than in Th1 cells) and in production of the IFN-γ-inducers, IL-12 and IL-18 within the host heart (Online Figure X). Administration of neutralizing antibody to IFN-γ after allotransplantation diminished systemic Th1 (but increased Th17) differentiation, reduced the levels of circulating IFN-γ, and decreased the expression of IFN-γ-inducers, IFN-γ, and IFN-γ-inducible molecules in the host heart (Figure 6A-E) as well as the donor graft (Online Figure X). As expected, neutralization of IFN-γ reduced the extent of arteriosclerosis in the vessels of the graft (Online Figure XI). Strikingly, anti-IFN-γ therapy also prevented the allograft-dependent increase in lipid deposition into and intimal thickening of the host aorta (Figure 7A-D) without causing a reduction in circulating lipids (Online Figure XI). Moreover, this treatment strategy prevented left ventricular dilatation, preserved left ventricular contractility, and restored aortic distension in allograft recipients (Figure 7E-H). Despite these benefits, neutralizing IFN-γ did not ensure survival of all hosts receiving allografts (Online Figure XI), although the experiment was not adequately powered to test for a partial improvement in survival.
Figure 6. Effects of IFN-γ neutralization on immune and inflammatory responses.
ApoE−/− recipients of syngeneic and class II-mismatched grafts were treated with irrelevant IgG or IFN-γ antibody from 0-12 wk post-op (A) Flow cytometry of IFN-γ and IL-17 expression by circulating CD4+ T cells. (B) Similar analyses in splenic CD4+ T cells. Cells for each analysis were pooled from 3 animals. (C) ELISA determination of IFN-γ plasma levels and (D) quantitative RT-PCR analysis of recipient hearts for IFN-γ and IFN-γ-inducible CXCL10 transcripts; n=3-5 per group, *P<0.05, ***P<0.001 vs. other groups. (E) Immunofluorescence analysis of native coronary arteries for the endothelial marker, CD31 (red color), for IFN-γ-inducible I-A/I-E MHC class II antigens (green color), and overlayed together with DAPI staining of nuclei (blue color), bars=50 μm. IFN-γ neutralization (lower panels) decreased inducible I-A/I-E antigen expression by arterial endothelial cells, but not the constitutive expression by perivascular leukocytes.
Figure 7. IFN-γ neutralization prevents graft-exacerbated host CVD.
ApoE−/− recipients of syngeneic or class II-mismatched grafts were treated with irrelevant IgG vs. neutralizing antibody to IFN-γ for 12 wk post-op. (A) Representative Oil Red O stains of host aortas and (B) expressed as % of total area. (C) Representative EVG stains of host aortic roots showing neointima (arrowheads), bar=200 μm and (D) intima area. (E) Left ventricular (LV) internal dimensions, (F) LV fractional shortening, (G) ascending aorta diameters, and (H) ascending aorta distension during the cardiac cycle derived from echocardiographic images of the host heart and aorta. n=3-6 per group, *P<0.05, **P<0.01 vs. other groups.
DISCUSSION
We find that pre-existent vascular disease and graft rejection are associated with accelerated atherosclerosis and de novo cardiovascular dysfunction in murine transplant recipients. Furthermore, IFN-γ produced by alloimmune responses and consequent inflammation play a non-redundant role in promoting the graft-exacerbated atherosclerosis, cardiomyopathy, and aortic stiffening of ApoE−/− hosts. The manifestations of CVD associated with graft rejection are qualitatively different than the enhanced atherosclerosis and modulation of plaque composition that has been described in hyperlipidemic mice with several other systemic inflammatory stimuli of autoimmune disease, microbial infection, and tissue injury29-31. In addition to greater lipid deposition, larger neointima formation, more extensive distribution, and increased mineralization of atherosclerotic lesions, we also find decreased ventricular contractility, ventricular dilatation, diminished contractile reserve, systolic hypertension, aortic stiffening, and unexplained deaths. This constellation of atherosclerosis and cardiovascular abnormalities resembles the skewed clinical presentation of graft-related CVD with accelerated heart failure and sudden death in renal transplant patients8,20-22.
By comparing hyperlipidemic to normolipidemic hosts, we conclude that pre-existent atherosclerosis due to conventional disease precipitants is necessary for the exaggerated inflammation and disease manifestations of the transplantation-induced phenotype. By varying donor-recipient genetic disparities and comparing different types of grafts, we conclude that both the strength and chronicity of alloimmune responses affect the pathogenesis of host CVD. Full-mismatched hearts are vigorously rejected within a relatively short period of time and alloimmune responses, deprived of antigen, begin to resolve after the graft is destroyed. Similarly, class II-mismatched skin grafts rapidly necrose due to failure of secondary vascularization. On the other hand, less vigorous rejection of class I- and minor-mismatched hearts is expected to delay the initiation of systemic inflammation in the host. The greatest degree of graft-exacerbated CVD (as indicated by significant coronary atherosclerosis and cardiac dysfunction) in recipients of class II-mismatched hearts may reflect the combination of intermediate strength, but relatively persistent alloresponses known to result in robust chronic graft rejection27. Alternatively, the prominent sequelae of class II-mismatched grafts may point to a pivotal role for CD4+ T helper cells, the lymphocyte subtype that directly recognizes and is activated by allogeneic MHC class II antigens and are, on a per cell basis, the major source of IFN-γ.
Our analysis of circulating cytokine levels at the termination of the experiments are not informative about peak levels or the duration of cytokine elevation and these limitations prevent a direct correlation to the extent of host CVD that develops over the entire post-op period. For example, IFN-γ plasma levels measured at 12 wk post-op may have been highest shortly after or during maximal rejection of single MHC antigen-mismatched grafts, whereas the concentrations may have been lower in full- and minor-mismatched recipients because cytokine production had already or not yet peaked, respectively. Serial measurements and the integration of cytokine levels over time may have provided a better correlate to host CVD, although repeated procedures can also influence the inflammatory state of the animals and confound the analysis. Additionally, the level of systemic inflammation may have been underestimated by the exclusion of animals with rejected allografts that died suddenly (possibly of cytokine storm) and did not allow for further serological analysis. Although our focus is on proinflammatory factors, we do not discount immunological perturbation of traditional metabolic and hemodynamic risk factors contributing to the pathogenesis of graft-exacerbated CVD in our model. Moderate hypertension (39% increase in mean blood pressure under conditions of anesthesia) ensued in recipients of class II-mismatched grafts at 12 wk post-op. However, this degree of blood pressure elevation is not thought to result in phenotypic changes in ApoE−/− mice32. Additionally, minor changes in circulating lipids that did not reach statistical significance are unlikely to account for the qualitative differences in CVD manifestations after allotransplantation.
We find evidence for activation of diverse immune responses in ApoE−/− transplant recipients, including of cell-mediated and humoral adaptive immunity, as is well described for the development of atherosclerosis in hyperlipidemic mice and for allograft rejection by normolipidemic hosts across the same strain combinations33-36. We extend these previous findings by demonstrating a marked enhancement of systemic inflammation, particularly of circulating CD4+ T cell activation, when the two models of disease are combined. Innate immune cells also play an important role in atherosclerosis and graft rejection37,38. The increased total number of macrophages with a skewed inflammatory phenotype within atherosclerotic lesions of ApoE−/− hosts may reflect activation by adaptive immune responses or by recognition of tissue damage signals in rejecting allografts similar to the injury effect seen with myocardial infarction of native hearts31. In this initial study, we focused on the role of T helper cells and IFN-γ that are crucial activators of many components of the immune system. Thus, other immune effectors dependent on CD4+ T cells and IFN-γ are also likely to contribute to graft-exacerbated CVD and will be the subject of future studies. The actions of T helper cells and IFN-γ in the pathogenesis of atherosclerosis have been extensively studied, as discussed below. However, we have not yet determined the exact mechanism(s) for cardiac failure and aortic stiffening. Even though IFN-γ is necessary for these pathological changes in our model, it may not be sufficient. Further work is required to determine if cardiovascular dysfunction is solely a consequence of atherosclerosis, i.e. following a continuum of occlusive arterial disease, myocardial infarction, and heart failure39. In other experimental systems, autoreactive T cells, autoreactive B cells and autoantibodies, and even individual cytokines can directly impair cardiac function independent of atherosclerotic disease40-42. Acute administration of endotoxin, a bacterial product that triggers an intense inflammatory response, is known to depress left ventricular systolic function independent of preload or afterload and result in greater systolic than diastolic left ventricular dilatation43. T cells and their cytokines may also result in stiffening of non-atherosclerotic aortas44 and there is clinical evidence that aortic stiffness may induce cardiac dysfunction45.
We postulate three mechanisms of disease in our model of graft-exacerbated CVD that are not mutually exclusive. First, systemic inflammation may enhance pro-atherosclerotic immune responses within the artery wall representing an endocrine effect of graft rejection on host disease. In support of this hypothesis, increased IL-6 levels have been found in patients with graft-related CVD18, though measures of other Th1- and Th17-related cytokines have not been reported in this particular clinical scenario. We have previously shown an inflammatory pathway of IFN-γ production by T cells in response to IL-12 and IL-18, independent of their cognate receptor interactions, in human coronary atherosclerosis46 and that IFN-γ is necessary and sufficient for the progression of human coronary arteriosclerosis25,47. IL-17, another key effector cytokine of adaptive immune responses, is produced concomitantly with IFN-γ by human coronary artery-infiltrating T cells with synergistic effects on vessel wall inflammation, but does not contribute to neointima formation48,49. Pro-atherosclerotic effects of IFN-γ and, controversially, of IL-17 have also been described in murine models50-53 and further work is required to dissect their interactions in graft-exacerbated CVD. Second, alloimmune responses between host and graft may directly damage recipient arteries via paracrine mechanisms. Host anti-graft T cells may re-circulate from the transplanted organ to the native heart and vasculature and contribute to pathological inflammatory responses, in particular if donor passenger leukocytes have taken residence in recipient tissues. Graft anti-host T cells are known to target the endothelium of transplant recipient microvessels causing clinical disease54 and similar unrecognized responses may occur in conduit arteries as these vessels are not generally sampled in diagnostic procedures. Third, graft rejection may induce autoimmune injury as the alloimmune response spreads to encompass antigens shared by the graft and the host. Of relevance, autoantibodies to nuclear and cytoplasmic self-antigens have been characterized in C57BL/6 recipients of bm12 cardiac grafts that result in donor artery injury, though effects on the normolipidemic host vasculature have not been described55. In the latter study, autoantibody production by recipient B cells was dependent on donor CD4+ T cell help and similar interactions may be operational for graft-exacerbated CVD of the host.
A limitation of our experimental system is the absence of immunosuppressive agents, unlike the clinical scenario of graft-related CVD. Effective immunosuppression in rodents readily leads to allograft acceptance with little residual immune responses. This may be because rodent models are primarily dependent upon naive T cell activation which is more easily suppressed than memory responses that dominate the early response to human allografts. Alternative approaches to obtain attenuated graft rejection by subtherapeutic doses or transient administration of drugs56 would invoke off-target effects on the host. Many immunosuppressive agents have unfavorable side effects on CVD risk factors and may promote atherosclerosis57. Conversely, immunosuppressants may inhibit the inflammation-driven progression of atherosclerosis58. We reasoned that avoiding confounding immunosuppression and depending on controlled immunogenetic differences were preferable for our initial studies. It may be that our experimental system best models the clinical situation in which immunosuppressants are discontinued after kidney graft failure and dialysis is reinitiated without excision of the donor organ (that is often contraindicated due to the risks of reoperative surgery in the inflamed site). With the reintroduction of immune competency, unrestrained alloimmune responses are expected and, significantly, are associated with a greater than 3-fold spike in CVD morbidity and mortality of renal transplant recipients59. Even after correcting for the selection bias of dialysis patients listed for transplantation, a deleterious effect for a failed allograft was revealed compared to no previous transplant60. Clinicians have focused attention on the chronic inflammatory state resulting from failed kidney allografts and some strongly recommend graft nephrectomy or continued use of immunosuppressive drugs in this setting, although there is no consensus for the management of this difficult problem61-63.
Medical advances have greatly improved the early, but not late outcomes after all types of organ transplantation. Chronic rejection and CVD have emerged as the leading obstacles to graft and patient survival. Both processes are characterized by arteriosclerotic lesions as well as ischemic and fibrotic sequelae64; both diseases represent risk factors for each other13-16. In our opinion, their concurrent manifestations in transplant recipients are not a coincidence, but represent a failure of current immunosuppressive regimens to prevent and treat these conditions - unlike the favorable results for acute rejection. The immunological mechanisms that contribute to the pathogenesis of native atherosclerosis and graft arteriosclerosis have been extensively studied. As discussed above, we and others have shown that adaptive immune responses, in particular that of Th1 cells, play a central role in both processes65,66. Thus, common pathogenetic mechanisms of excessive IFN-γ activity on vessel wall cells may link graft arteriosclerosis and host CVD. While our study adds to the rationale for neutralizing IFN-γ as part of the management of transplant recipients, the unexplained failure of this therapy to prevent all host deaths in our model despite improved cardiovascular outcomes indicates that the adverse effects of having chronic immune stimulation caused by the presence of allogeneic tissue is likely to be more complex than the effects of increased levels of a single cytokine.
In conclusion, our experimental model replicates many aspects of a disease process that represents a major obstacle to the long-term success of organ replacement therapy. It provides a preclinical framework to define hitherto unsuspected disease mechanisms and to test new therapeutic approaches to prevent or reverse graft-related CVD of the transplant recipient. Furthermore, our work identifies IFN-γ as a therapeutic target to inhibit CVD of the host in addition to previous studies that have validated IFN-γ antagonism as treatment for arteriosclerosis of the graft.
Supplementary Material
Novelty and Significance.
What Is Known?
Organ transplant recipients have an increased risk of cardiovascular disease (CVD) and premature death.
The increased occurrence of CVD and its complications is only partially accounted by traditional risk factors.
The restricted predictive value of traditional risk factors for CVD in organ transplant recipients is interpreted to indicate the effect of additional, unidentified disease precipitants.
What New Information Does This Article Contribute?
Graft rejection in hyperlipidemic mice exacerbates atherosclerosis and induces de novo dysfunction of the recipient heart.
Increased atherosclerosis and cardiovascular dysfunction of the host is associated with systemic and local activation of the immune system, particularly of T helper cells and the production of interferon-γ.
Neutralization of interferon-γ prevents the worsening of atherosclerosis and the onset of cardiovascular dysfunction in hosts with rejecting grafts.
Solid organ transplantation is life-saving, but may cause disease and death of the recipient if it fails, is rejected, or, in rare circumstances, transmits malignant cells, pathogens, or passenger leukocytes sufficient for graft-versus-host disease. A detrimental effect of the graft on the host cardiovascular system has not been suspected even though graft dysfunction is an independent risk factor for CVD in organ transplant recipients. In hyperlipidemic mice, we find that intra-abdominal graft rejection is associated with increased atherosclerosis and dysfunction of the host heart and aorta. This disease association across body cavities is characterized by systemic and local activation of leukocytes and the production of cytokines, particular of T helper cells and interferon-γ. Antibody neutralization of interferon-γ prevents the exacerbated host atherosclerosis and cardiovascular dysfunction. Our findings suggest that graft-related inflammation in organ transplant recipients may worsen their CVD and lead to their premature death. In addition to the known benefits of inhibiting interferon-γ responses in native atherosclerotic disease and in chronic allograft vasculopathy, we identify interferon-γ as a therapeutic target in graft-exacerbated CVD of the host. Our data provide a rationale for extending ongoing treatment trials with anti-interferon-γ antibody from patients with autoimmunity to allograft recipients.
Acknowledgments
SOURCES OF FUNDING
This work was supported by an intramural Ohse grant and an American Heart Association Scientist Development Grant (13SDG17170019 to J.Z.), by the National Institutes of Health (RO1-HL109455 to J.S.P.), and by Yale University Department of Surgery and Section of Cardiac Surgery (G.T.).
Nonstandard Abbreviations and Acronyms
- ApoE−/−
apolipoprotein E-deficient
- CVD
cardiovascular disease
- D
day
- EVG
elastin Van Gieson
- H&E
hematoxylin and eosin
- H
hour
- IFN
interferon
- IL
interleukin
- MHC
major histocompatibility complex
- Min
minute
- Op
operative
- Sc
subcutaneous
- Wk
week
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11:1226–35. doi: 10.1111/j.1600-6143.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 2.Lindholm A, Albrechtsen D, Frödin L, Tufveson G, Persson NH, Lundgren G. Ischemic heart disease--major cause of death and graft loss after renal transplantation in Scandinavia. Transplantation. 1995;60:451–7. doi: 10.1097/00007890-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–13. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Matas AJ, Humar A, Gillingham KJ, Payne WD, Gruessner RW, Kandaswamy R, Dunn DL, Najarian JS, Sutherland DE. Five preventable causes of kidney graft loss in the 1990s: a single-center analysis. Kidney Int. 2002;62:704–14. doi: 10.1046/j.1523-1755.2002.00491.x. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 6.Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, Zein NN. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370–5. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 7.Takayama H, Salerno CT, Aldea GS, Verrier ED. Characteristics of extracoronary vascular disease in heart transplant recipient. J Card Surg. 2008;23:459–63. doi: 10.1111/j.1540-8191.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- 8.Holdaas H, Fellström B, Jardine AG, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–31. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11:1735–43. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 10.Jardine AG, Fellström B, Logan JO, Cole E, Nyberg G, Grönhagen-Riska C, Madsen S, Neumayer HH, Maes B, Ambühl P, Olsson AG, Pedersen T, Holdaas H. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis. 2005;46:529–36. doi: 10.1053/j.ajkd.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Kiberd B, Panek R. Cardiovascular outcomes in the outpatient kidney transplant clinic: the Framingham risk score revisited. Clin J Am Soc Nephrol. 2008;3:822–8. doi: 10.2215/CJN.00030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver SA, Huang M, Nash MM, Prasad GV. Framingham risk score and novel cardiovascular risk factors underpredict major adverse cardiac events in kidney transplant recipients. Transplantation. 2011;92:183–9. doi: 10.1097/TP.0b013e31821f303f. [DOI] [PubMed] [Google Scholar]
- 13.Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, Kasiske BL, PORT Investigators Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant. 2010;10:338–53. doi: 10.1111/j.1600-6143.2009.02949.x. [DOI] [PubMed] [Google Scholar]
- 14.Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet. 2011;378:1419–27. doi: 10.1016/S0140-6736(11)61334-2. [DOI] [PubMed] [Google Scholar]
- 15.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7:158–65. doi: 10.1681/ASN.V71158. [DOI] [PubMed] [Google Scholar]
- 16.Fellström B, Jardine AG, Soveri I, Cole E, Neumayer HH, Maes B, Gimpelewicz C, Holdaas H, ALERT Study Group Renal dysfunction is a strong and independent risk factor for mortality and cardiovascular complications in renal transplantation. Am J Transplant. 2005;5:1986–91. doi: 10.1111/j.1600-6143.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- 17.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 18.Abedini S, Holme I, März W, Weihrauch G, Fellström B, Jardine A, Cole E, Maes B, Neumayer HH, Grønhagen-Riska C, Ambühl P, Holdaas H, ALERT study group Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4:1246–54. doi: 10.2215/CJN.00930209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinkeler SJ, Zelle DM, Homan van der Heide JJ, Gans RO, Navis G, Bakker SJ. Endogenous plasma erythropoietin, cardiovascular mortality and all-cause mortality in renal transplant recipients. Am J Transplant. 2012;12:485–91. doi: 10.1111/j.1600-6143.2011.03825.x. [DOI] [PubMed] [Google Scholar]
- 20.Holdaas H, Fellström B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Grönhagen-Riska C, Neumayer HH, Maes B, Ambühl P, Hartmann A, Staffler B, Jardine AG, Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005;5:2929–36. doi: 10.1111/j.1600-6143.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 21.Rigatto C, Foley R, Jeffery J, Negrijn C, Tribula C, Parfrey P. Electrocardiographic left ventricular hypertrophy in renal transplant recipients: prognostic value and impact of blood pressure and anemia. J Am Soc Nephrol. 2003;14:462–8. doi: 10.1097/01.asn.0000043141.67989.39. [DOI] [PubMed] [Google Scholar]
- 22.Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, Brennan DC. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis. 2005;46:720–33. doi: 10.1053/j.ajkd.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Rigatto C. Clinical epidemiology of cardiac disease in renal transplant recipients. Semin Dial. 2003;16:106–10. doi: 10.1046/j.1525-139x.2003.16026.x. [DOI] [PubMed] [Google Scholar]
- 24.Verbeke F, Van Biesen W, Peeters P, Van Bortel LM, Vanholder RC. Arterial stiffness and wave reflections in renal transplant recipients. Nephrol Dial Transplant. 2007;22:3021–7. doi: 10.1093/ndt/gfm379. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Burns WR, Tang PC, Yi T, Schechner JS, Zerwes HG, Sessa WC, Lorber MI, Pober JS, Tellides G. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. FASEB J. 2004;18:606–8. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- 26.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–7. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 27.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. I. Time course and immunogenetic and immunopathological considerations. Am J Pathol. 1994;144:260–74. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–25. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanic AK, Stein CM, Morgan AC, Fazio S, Linton MF, Wakeland EK, Olsen NJ, Major AS. Immune dysregulation accelerates atherosclerosis and modulates plaque composition in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2006;103:7018–23. doi: 10.1073/pnas.0602311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs P, Scandella E, Bolinger B, Engeler D, Miller S, Ludewig B. Chronic immune reactivity against persisting microbial antigen in the vasculature exacerbates atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 2007;27:2206–13. doi: 10.1161/ATVBAHA.107.141846. [DOI] [PubMed] [Google Scholar]
- 31.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Cassis LA, Daugherty A. Atherosclerosis and arterial blood pressure in mice. Curr Drug Targets. 2007;8:1181–9. doi: 10.2174/138945007782403829. [DOI] [PubMed] [Google Scholar]
- 33.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. III. Effects of recipient treatment with a monoclonal antibody to interferon-gamma. Transplantation. 1994;57:1367–71. [PubMed] [Google Scholar]
- 34.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152:5135–41. [PubMed] [Google Scholar]
- 35.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–22. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 36.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–53. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003;111:1571–8. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114:2850–70. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 40.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188:4876–84. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Tassell BW, Seropian IM, Toldo S, Mezzaroma E, Abbate A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62:637–40. doi: 10.1007/s00011-013-0625-0. [DOI] [PubMed] [Google Scholar]
- 43.Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–7. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–25. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 46.Ranjbaran H, Sokol SI, Gallo A, Eid RE, Iakimov AO, D'Alessio A, Kapoor JR, Akhtar S, Howes CJ, Aslan M, Pfau S, Pober JS, Tellides G. An inflammatory pathway of IFN-gamma production in coronary atherosclerosis. J Immunol. 2007;178:592–604. doi: 10.4049/jimmunol.178.1.592. [DOI] [PubMed] [Google Scholar]
- 47.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–11. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 48.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–58. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Pablo AM, Jiang Xc, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–61. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–24. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–75. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 53.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–55. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, Gratwohl A. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–83. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 55.Win TS, Rehakova S, Negus MC, Saeb-Parsy K, Goddard M, Conlon TM, Bolton EM, Bradley JA, Pettigrew GJ. Donor CD4 T cells contribute to cardiac allograft vasculopathy by providing help for autoantibody production. Circ Heart Fail. 2009;2:361–9. doi: 10.1161/CIRCHEARTFAILURE.108.827139. [DOI] [PubMed] [Google Scholar]
- 56.George JF, Pinderski LJ, Litovsky S, Kirklin JK. Of mice and men: mouse models and the molecular mechanisms of post-transplant coronary artery disease. J Heart Lung Transplant. 2005;24:2003–14. doi: 10.1016/j.healun.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto Y, Hof A, Baumlin Y, Hof RP. Differential effect of cyclosporine A and SDZ RAD on neointima formation of carotid allografts in apolipoprotein E-deficient mice. Transplantation. 2003;76:1166–70. doi: 10.1097/01.TP.0000090393.75600.32. [DOI] [PubMed] [Google Scholar]
- 58.Elloso MM, Azrolan N, Sehgal SN, Hsu PL, Phiel KL, Kopec CA, Basso MD, Adelman SJ. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am J Transplant. 2003;3:562–9. doi: 10.1034/j.1600-6143.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2:970–4. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 60.Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294–300. doi: 10.1053/j.ajkd.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Ayus JC, Achinger SG. At the peril of dialysis patients: ignoring the failed transplant. Semin Dial. 2005;18:180–4. doi: 10.1111/j.1525-139X.2005.18304.x. [DOI] [PubMed] [Google Scholar]
- 62.Marcén R, Teruel JL. Patient outcomes after kidney allograft loss. Transplant Rev. 2008;22:62–72. doi: 10.1016/j.trre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Gill JS. Managing patients with a failed kidney transplant: how can we do better? Curr Opin Nephrol Hypertens. 2011;20:616–21. doi: 10.1097/MNH.0b013e32834bd792. [DOI] [PubMed] [Google Scholar]
- 64.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–15. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 65.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–32. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 66.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.