Abstract
Ethnopharmacological relevance
Nelumbo nucifera Geartn., known as sacred lotus, has been used traditionally in South East Asia as a traditional medicine for various CNS disorders including stress, fever, depression, insomnia, and cognitive conditions.
Aim of the study
To investigate the in vitro cannabinoid and opioid receptor binding affinities, and in vivo behavioral actions of Nelumbo flower extracts and to isolate the potential compounds to treat CNS associated disorders.
Materials and methods
The white and pink flowers of N. nucifera were extracted with 95% EtOH, followed by acid-base partitioning using CHCl3 to give acidic and basic partitions. These partitions were subjected to Centrifugal Preparative TLC (CPTLC) to yield benzyltetrahydroisoquinoline (BTIQ) alkaloids and long chain fatty acids, identified by physical and spectroscopic methods. In addition, EtOH extracts and partitions were analyzed for chemical markers by UHPLC/MS and GC/MS. In vitro neuropharmacological effects were evaluated by cannabinoid (CB1 and CB2) and opioid [delta (δ), kappa (κ), and mu (μ)] competitive radioligand binding and GTPγS functional assays. The in vivo behavioral effect was studied through the use of the mouse tetrad assay at 10, 30, 75 and 100 mg/kg/ip doses that revealed the effect on locomotion, catalepsy, body temperature, and nociception of acidic and basic CHCl3 partitions, fractions, and compounds.
Results
Three aporphines, nuciferine (1), N-nor-nuciferine (2), asimilobine (3), and five BTIQs, armepavine (4), O-methylcoclaurine (5), N-methylcoclaurine (6), coclaurine (7), neferine (10), and a mixture of linoleic and palmitic acids (LA and PA), were identified and evaluated for cannabinoid and opioid receptor displacement activities. Compounds 5–7 showed binding affinities for the κ opioid receptor with equilibrium dissociation constant (Ki) values of 3.5±0.3, 0.9±0.1, 2.2±0.2 µM, respectively. Compound 10 displayed affinities for δ-and μ- opioid receptors with Ki values of 0.7±0.1 and 1.8±0.2 µM, respectively, and was determined to be a weak δ agonist by GTPγS functional assay. The mixture of LA and PA (1:1) showed an affinity for δ opioid receptor with a Ki value of 9.2±1.1 µM. The acidic and basic CHCl3 partitions, compounds 1 and 7, and 5–7 mixture were subjected to the tetrad assay, of which the acidic partition displayed decreased locomotion and increased catalepsy, antinociception, and hypothermia in animal at doses of 75–100 mg/kg/ip, and also showed clonic-tonic seizures upon touch at 100 mg/kg.
Conclusion
Bioassay-guided isolation revealed compounds 5–7, 10, and the mixture of LA and PA displayed various degrees of opioid receptor radioligand displacement affinities. The in vivo tetrad assay of acidic CHCl3 partition, enriched with aporphines 1 and 2, displayed actions on all four points of behavioral parameters. It can be concluded that the in vivo mild canabimimetic-type effect observed for the CHCl3 partition is likely mediated through other CNS mechanisms since the extracts, partitions, and isolated compounds had no affinity for the in vitro CB1 and CB2 receptors. This work, along with traditional use and the reported bioactivities of the BTIQ alkaloids, suggested further studies on N. nucifera are needed to understand the roles that the extracts and/or individual compounds might contribute to the behavioral effects.
Keywords: Nelumbo nucifera; alkaloids; chromatography; central nervous system; cannabinoid (CB1, CB2); opioid receptors (δ, κ, μ)
1. Introduction
Nelumbo nucifera Gaertn. (Nymphaeaceae), known as sacred lotus, Chinese water lily or water lotus, is widely distributed throughout South-East Asia. In many Asian countries the aquatic plant has been reported to have a wide array of traditional, medicinal and therapeutic uses (Duke et al., 2002; Mehta et al., 2013; Mitra and Kapoor, 1976; Nakamura et al., 2013). In traditional Chinese medicine, seed embryos (or plumule) of the flower are used to overcome nervous disorder, high-fever with restlessness, and insomnia (Mehta et al, 2013; Nguyen, 1999) and arrow-root prepared from the rhizome “OCE FUN“ for enhancing the mental health and quieting the spirits (Porterfield, 1951). Furthermore, N. nucifera has been utilized as ingredient for healthy beverages to treat hypertension, cancer, weakness and body heat imbalance (Saengkhae et al., 2008), and also smoking the plant create a feeling of well-being, controlling the stress and reaching a relaxing state (O’Mahony Carey, 2010). However, the traditional use of N. nucifera has been significantly correlated to several central nervous system (CNS) disorders including stress, depression, pain and cognitive disorders (Chiang Su, 1978; Mathew and Subramanian, 2014; Kang et al., 2005; Tanahashi et al., 2006; Nakajima et al., 2007). A detailed chemical and neuropharmalogical studies of N. nucifera flower extracts has not yet been undertaken, but earlier chemical studies on other parts of the plant reported the presence of alkaloids, triterpenoids, flavonoids, steroids, polyphenols, fatty acids, and glycosides (Buddhadev and Buddhadev, 2014; Mukherjee et al., 1996). In addition, the in vivo psychopharmacological effects of rhizome extracts, as well as analgesic and antipyretic activity, have also been reported (Mukherjee et al., 2009; Nakamura et al., 2013). A recent study on N. nucifera petal extract revealed significant agonist and antagonist activity towards serotonin (5-HT2C) and cannabinoid receptor 2, respectively, indicating its role in obesity as an appetite suppressant (Velusami et al., 2013). In addition, in vivo studies demonstrated hypothermic, sedative, and analgesic effect of N. nucifera embryo extract, and sedative and antidepressant effect of neferine (Nakajima et al., 2007), neuroleptic acvtivity of nuciferine (Bhattacharya et al., 1978), sedative effect of N-methylcoclaurine, and antidepression-like effect of armepavine (Tanahashi et al., 2006).
Cannabinoid and opioid receptors are G-protein coupled receptors, and are involved in the management of various cellular, neuronal, and cardiovascular functions, including addiction, pain, fever, and neurological disorders. The opioid peptides and receptors regulate a variety of neurophysiologic functions, including pain control. Nevertheless, long-term use of currently available drugs for treating neuropsychiatric diseases has resulted in various undesirable side effects leading to drowsiness, addiction, and far-reaching health consequences. Medicinal plants with milder CNS activities are increasingly used as a supplement to treat a number of CNS disorders including pain, anxiety, convulsions, epilepsy, hysteria, nightmares, and many other disorders. As part of our program to study the chemical and neuropharmacological activity of medicinal plants, the basic CHCl3 partition of EtOH extracts of N. nucifera flower showed strong in vitro κ- (kappa) and μ- (mu) opioid receptor displacements (95.2% and 95.8%, respectively), while the acidic partition was found to be inactive against these receptors. Bioassay-guided isolation of basic partition afforded seven alkaloids, namely N-nor-nuciferine (2) and asimilobine (3) (Shoji et al., 1987; Yang et al., 2014), armepavine (4) (Nakamura et al., 2013), O-methylcoclaurine (5) and N-methylcoclaurine (6) (Mukherjee et al., 2009) and coclaurine (7) (Kashiwada et al., 2005). In addition, nuciferine (1), additional amounts of 2, linoleic and palmitic acids (LA and PA) (Zhao et al., 2013), and stigmasterol were isolated from acidic partition. In this study, we report the isolation, structure elucidation, analog preparation, and evaluation of in vitro CB and opioid receptor displacement activities and behavioral studies using in vivo mouse tetrad assay of extracts, partitions, and isolated compounds.
2. Materials and methods
2.1 General experimental procedures
Optical rotations were measured in CHCl3 or MeOH using an AUTOPOL IV® polarimeter at room temperature. The NMR spectra were acquired on a Bruker Avance DRX-400 instrument at 400 MHz (1H), 100 (13C) in CDCl3 or CD3OD, using the residual solvent as internal standard; multiplicity determinations (DEPT) and 2D NMR spectra (COSY, HMQC, and HMBC) were obtained using standard Bruker pulse programs. HRMS were obtained by direct injection using a Bruker Bioapex-FTMS with electrospray ionization (ESI). The UHPLC-DAD-MS data were obtained from an Agilent 1290 Infinity series UHPLC with a diode array detector and an Agilent 6120 quadrupole mass spectrometer. Gas chromatographic analysis was performed on an Agilent 7890 GC instrument equipped with an Agilent 7693 auto-sampler. Analytical TLC was carried out on silica gel (EMD® Chemicals Inc., SiO2 60 F254) or alumina plates (EMD® Chemicals Inc., Al2O3 60 F254). Centrifugal preparative TLC (CPTLC) was performed by a Chromatotron® (Harrison Research Inc., model 8924), tagged with a fraction collector (SpectralChrom® CF-1) on a 3 mm and 6 mm customized Al2O3 Chromatorotors; (1092-1) 60 GF254 Neutral l type E Al2O3 was used as the sorbent with F254 and UV366 indicators. Samples were dried using a Savant Speed Vac Plus SC210A Concentrator. The isolated compounds were visualized by observing under UV light at 254 or 365 nm, followed by spraying separately with Dragendorff’s reagent for alkaloids and/or 1% vanillin-H2SO4 spray reagents for non alkaloids. The reference standards neferine, linoleic acid, and palmitic acid were purchased from Sigma-Aldrich (St. Louis, MO). Higenamine was purchased from Health Supplement Wholesalers LLC (East Market Street, Suite G, York, PA 17402-2861). Buffer reagents were purchased from Sigma-Aldrich (St. Louis, MO). All radioligands and MicroScint20 were purchased from PerkinElmer (Waltham, MA). Non-labeled controls were purchased from Tocris Bioscience (Minneapolis, MN).
2.2 Extraction of plant material
2.2.1 Plant material
The white and pink flowers of N. nucifera were purchased from Bouncing Bear Botanicals (P.O. Box 1993, Lawrence, KS 66044) in November, 2013, and the reference voucher specimens (NCNPR # 15038 and # 15045, respectively) were prepared by Dr. Vijayasankar Raman, and deposited at the National Center for Natural Products Research, School of Pharmacy, University of Mississippi. Samples used in this work were preserved using the standard procedures for procurement, processing and packaging at the National Center for Natural Products Research. The supplier has informed that the samples were originated from Thailand.
2.2.2 Preparation of extracts
The dried white flowers (1 kg) were grounded, and extracted successively by maceration with 95% EtOH (1:2; w/v × 4 times) at room temperature. Filtrates of each extract were combined and dried over a rotary evaporator at 40 °C to yield a dried gummy residue (44 g), which was submitted for CB (subtypes CB1 and CB2) and opioid (subtypes δ, κ, μ) competitive radioligand binding assays.
2.3 Bioassay-guided isolation and identification of compounds
The EtOH extracts of the white and pink flowers of N. nucifera, displayed 46% and 54% displacement of the radioligand for the κ opioid receptor, respectively. A portion of the extract of white flower (42 g) was dissolved in 0.1 N HCl and partitioned with CHCl3 (3 times) to give the acidic CHCl3 partition (640 mg). The acidic aqueous layer was then basified with 30% NH4OH (pH 11) and partitioned with CHCl3 (3 times) to yield the basic CHCl3 partition (1.5 g). The basic fraction (1.4 g) was chromatographed over a CPTLC, using a 6 mm customized Chromatorotor™ (Muhammad et al., 2013) packed with neutral alumina, using a Chromatotron®, and eluted with CH2Cl2-MeOH (99:1 to 90:10) to yield N-nor-nuciferine (2, 58 mg), asimilobine (3, 80 mg), armepavine (4, 31 mg), O-methylcoclaurine (5, 28 mg), N-methylcoclaurine (6, 46 mg), and coclaurine (7, 65 mg). The acidic fraction (236 mg) was subjected to CPTLC, using a 2 mm customized alumina Chromatorotor™ with CH2Cl2-MeOH (99:1) as solvent, to afford the nuciferine (1, 60 mg) and additional amounts of 2 (13 mg). In addition, a portion of the acidic fraction (200 mg) was subjected to CPTLC, over a 2 mm silica gel Chromatorotor™, using n-hexanes-CH2Cl2 as solvent, to yield linoleic acid (15 mg), mixture of linoleic and palmitic acid (35 mg, fractions G and H), a fatty alcohol (20 mg) and stigmasterol (24 mg). A similar extraction and isolation procedure was adopted for the pink flowers of N. nucifera (750 g), which yielded EtOH extract (18.7 g), acidic CHCl3 partition (190 mg) and basic CHCl3 partition (283 mg).
Nuciferine (1): [α]D23.5 −172 (c 0.15, MeOH); UHPLC/APCI-MS m/z 296.2 ([M+H]+) C19H21NO2; The 1H and 13C NMR were indistinguishable to those reported (Zhenjia et al., 2010).
N-nor-nuciferine (2): [α]D23.5 −125 (c 0.15, MeOH); UHPLC/APCI-MS m/z 282.2 ([M+H]+) C18H19NO2+H; The 1H and 13C NMR were indistinguishable to those reported (Zhenjia et al., 2010).
Asimilobine (3): [α]D23.5 −256 (c 0.15, CHCl3). UHPLC/APCI-MS m/z 268.3 ([M+H]+) C17H17NO2; The 1H and 13C NMR were indistinguishable to those reported (Rollinger et al., 2006).
Armepavine (4): [α]D23.5 +39.9 (c 0.1, CHCl3).UHPLC/APCI-MS m/z 314.2 ([M+H]+) C19H23NO3; The 1H and 13C NMR were indistinguishable to those reported (Orejarena Pacheco et al., 2013).
O-Methylcoclaurine (5): [α]D23.5 +4.36 (c 0.4, MeOH); UHPLC/APCI-MS m/z 300.2 ([M+H]+) C18H21NO3; The 1H and 13C NMR were indistinguishable to those reported (Tanahashi et al., 2006).
N-Methylcoclaurine (6): [α]D23.5 −11.19 (c 0.25, CHCl3); UHPLC/APCI-MS m/z 300.4 ([M+H]+) C18H21NO3,; 1H NMR (CDCl3 400 MHz) : δH 2.44 (3H, s, N-CH3), 2.61 (1H, m, H-4,), 2.80 (2H, m, H3, H-4a), 2.74 (1H, m, H-α), 3.03 (1H, dd, J = 12.0, 4.0 Hz, H-α), 3.23 (1H, m, H-3), 3.71 (1H, t, J=4Hz, H-1), 3.29 (3H, s, O-CH3), 6.29 (1H, s, H-5), 6.51 (1H, s, H-8), 6.55 (2H, d, J=8 Hz, H1, H-13), 6.87 (2H, d, J=8 Hz, H10, H-14). 13C NMR (CDCl3, 100 MHz): δC 24.5 (C-4), 40.4 (C-1), 41.9 (C-3), 46.1 (C-2), 55.9 (O-CH3), 64.8 (O-CH3), 111.0 (C-5), 114.5 (C-8), 115.7 (C-3´ and C-5´), 124.3 (C-4a), 129.3 (C-8a), 130.3 (C-1´), 130.5 (C-2´ and C-6´), 143.6 (C-7), 146.0 (C-6), 155.2 (C-4´).
Coclaurine (7): [α]D23.5 +0.96 (c 0.31, MeOH). UHPLC/APCI-MS m/z 286.2 ([M+H]+) C17H19NO3; The 1H and 13C NMR were indistinguishable to those reported (Asencio et al., 1993).
Methylation of (±)-higenamine (dl-demethylcoclaurine) (11): The commercial sample of higenamine (100 mg) was purified by dissolving in MeOH to remove the insoluble precipitate, and the filtrate was evaporated to give racemic (±)-higenamine (20 mg); [α]D23.5 (±)-0 (c 0.15, MeOH). The structure of higenamine was confirmed by its spectroscopic data and by comparison with a reference standard available in our labs. Higenamine (50 mg) was dissolved in anhydrous methanol (2 mL) and methylated using trimethylsilyldiazomethane (1.0 mL) and the reaction mixture was kept at room temperature for 24 hours. Two major products were observed by TLC and separated by CPTLC using a 2 mm custom Chromatorotor™, over silica gel, and eluted with CH2Cl2-MeOH to yield racemic 12 (4´-O-methylarmepavine, 2.0 mg; [α]D23.5 (±)-0 (c 0.1, MeOH) and 13 (4´,7-di-O-methylarmepavine, 1.6 mg; [α]D23.5 (±)-0 (c 0.1, MeOH).
2.3.1 UHPLC/MS analysis
The UHPLC-DAD-MS data were obtained from an Agilent 1290 Infinity series UHPLC with a diode array detector and an Agilent 6120 quadrupole mass spectrometer. The mass spectrometer contained a dual APCI and ESI interface. The LC Column was a Waters Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm). The column temperature was 30 °C. The flow rate was 0.3 mL/minute. The mobile phase eluent consisted of acetonitrile and water both containing 0.05% formic acid. The gradient was 5% acetonitrile programmed to 80% acetonitrile in 15 minutes, and then 100% in 20 minutes. Ionization and detection of compounds were carried out on the mass spectrometer using the ESI positive mode over the mass range of m/z 100–1000. The fragmentor and capillary voltages were 120 V and 4000 V, respectively. The drying gas flow rate was 10.0 L/minute, the nebulizer pressure was 30 psi, and the drying gas temperature was 300 °C. The DAD was set to monitor 254 nm, 280 nm, and 325 nm.
2.3.2 GCMS analysis
About 1 mg of the dried EtOH extracts of white and pink N. nucifera flower were extracted with 450 µL of methylene chloride. Fifty microliter of trimethylsulfonium hydroxide (TMSH) was added to the solution for the methylation of the fatty acids, LA and PA.
Gas chromatographic analysis was performed on an Agilent 7890 GC instrument equipped with an Agilent 7693 auto-sampler. A fused silica capillary column (30 m, 0.25 mm i.d.) coated with a 0.20 µm film of cross-linked 5% diphenyl and 95% dimethylpolysiloxane (Agilent J&W HP-5MS) was used with helium as the carrier gas at a flow rate of 1 mL/minute. In a typical analysis, the oven was held for 2 minutes at 80 °C, and then programmed at 5 °C /minute to 170 °C, 2 °C / minute to 190 °C, 8 °C / minute to 280 °C held for 5 minutes The injector temperature was kept at 260 °C. The split ratio was 25:1.
Mass spectrometric analysis was carried out with an Agilent 5975C mass specific detector. The instrument was operated in scan mode, and the spectra were recorded at 70eV from m/z 40 to 500. Ions of m/z 67 and 74 were selected for extracted ion chromatography for LA and PA methyl esters, respectively. Compound identification involved comparison of the spectra with the databases (Wiley and NIST) using a probability-based matching algorithm. Further identification was based on the retention times compared with reference standards. The peak area percentages were calculated for LA and PA methyl esters.
2.4 Cannabinoid and opioid receptor radioligand binding assay
2.4.1 Cell Culture
HEK293 cells (ATCC) were stably transfected with cannabinoid receptor subtypes 1 and 2 and maintained as described in methods previously published (Husni et al., 2014). HEK293 cells stably transfected with δ-, κ-, and μ- opioid subtypes were a generous gift from Roth Laboratories (University of North Carolina at Chapel Hill, N.C., USA). Cell lines were maintained as previously described (Giacometti et al., 2013; Journigan et al., 2014).
2.4.2 Membrane preparation
Membranes were made by washing the cells with cold PBS. The cells were then scraped in cold 50 mM Tris-HCl buffer, pH 7.4. The solution was centrifuged at 5,200 × g for 10 minutes at 4 °C. The supernatant was discarded and the pellet was washed with more Tris-HCl buffer, sonicated via a Sonic Dismembrator Model 100 (Fisher Scientific, Pittsburgh, PA) and then centrifuged at 24,000 × g for 40 minutes at 4 °C. The pellet was re-suspended in Tris-HCl buffer to a desired concentration using a Pierce™ BCA Protein Assay according to manufacturer’s instructions. The isolated membrane was aliquoted into 2 mL vials and stored at −80 °C.
2.4.3. Competitive radioligand binding assays
Cannabiniod and opioid competitive radioligand binding assays were performed as previously described (Giacometti et al., 2013; Husni et al., 2014; León et al. 2013; Tarawneh et al., 2013). Membrane evaluations and saturation experiments were performed for all receptors to determine optimal receptor concentration (varied from 1 µg to 25 µg protein) and radioligand dissociation constant (Kd) for the membrane (varied between 0.5 nM to 5 nM).
Bioassay-guided isolation was performed through a screening process where different extracts, fractions, and purified compounds were evaluated at a fixed concentration (10 µg /mL, 10 µg /mL, and 10 µM, respectively) in triplicate for each cannabinoid and opioid subtype. The samples competed with a tritium-labeled ligand with a known affinity of the receptor of interest {[3H]-CP-55,940 (CB1 and CB2); [3H]-U-69,593 (κ); [3H]-DAMGO (μ); [3H]-Enkephalin (DPDPE) (δ)}.
Cannabinoid samples were incubated for 90 minutes at 37 °C with gentle agitation in 50 mM TrisHCl, 20 mM EDTA, 154 mM NaCl, 0.2% BSA, pH 7.4, and filtered through GF/C filterplates using a Perkin Elmer FilterMate Harvester. The assay plate was washed 10× with ice cold 50 mM TrisHCl, pH 7.4, 0.1% BSA. Opioid samples were incubated for 60 minutes at room temperature in 50 mM TrisHCl, pH 7.4, and filtered through GF/B filterplates which were washed 10× with ice cold 50 mM Tris-HCl buffer, pH 7.4.
Radioactivity was quantified on a TopCount NXT Microplate Scintillation counter after the addition of MicroScint20 to the dried filterplate. Percent displacement was calculated to represent the ability of the samples to displace the radioligand binding for a given cannabinoid or opioid receptor subtype.
If a purified compound exhibited % displacement > ~50% then a competitive radioligand binding assay was performed with a serial dilution of independent triplicate dilutions between 0.0017–300 µM for the test compound and between 0.017–3,000 nM for control compounds. The assays were tested with the same conditions stated above. The IC50 and Ki values were calculated by a non-linear curve fit model using GraphPad Prism 5.0 software (GraphPad Software, San Diego California, USA).
2.4.4 In vitro GTPγS functional assay
The GTPγS function assay was performed as previously described with slight modifications (Gao et al., 2013; Husni et al., 2014). The assay was preformed with 10–12 triplicate concentrations of each compound ranging from 0.003–500 µM. Dilutions for the GTPγS assay were made using GTPγS assay buffer (50 mM Tris-HCl, 150 mM NaCl, 9 mM MgCl2, 0.2 mM EGTA, 1.4 mg/mL essentially fatty acid free BSA). For each assay, non-specific binding was determined using 40 µM of non-labeled GTPγS salt, the basal control was determined in the presence of the vehicle only and Emax was determined using 10 µM of nonlabeled DPDPE (δ-opioid receptors) or 10 µM of non-labeled DAMGO (μ-opioid receptors). Each 250 µL test well contained 0.05 nM of [35S]-Guanosine 5’-(γ-thio) Triphosphate (PerkinElmer, Waltham, MA), compound, control agonist, or vehicle, 10 µM GDP, and 25 µg cell membrane. The assays were incubated for 60 minutes at room temperature. Percent stimulation from basal control was calculated by subtracting the mean basal control from the each value obtained in the presence of a ligand and then dividing the change by the mean basal control. The EC50 values were calculated by a non-linear curve fit model using GraphPad Prism 5.0 software.
2.5 Pharmacological experiments
Mouse tetrad assay was carried out using published method (Martin et al., 1991, 1994).
2.5.1 Animal
Adult male Swiss Webster mice (21–24 g) were obtained from Harlan Laboratories. All animals were housed five to a cage and received food/water ad lib. The housing facilities were maintained on a 12 h light/dark schedule (lights on at 6:00 am and off at 6:00 pm). Mice were randomly divided into 3 groups (n=10/dose).
2.5.2 Mouse tetrad assay
The mouse tetrad is a behavioral assay developed by Martin et al. (1994) to characterize the biological effects of cannabinoids using locomotor activity, nociception, changes in body temperature, and catalepsy. The assay has been well documented to indicate that the typical effects of cannabinoids is decreased in locomotion, and increased in cataleptic activity, antinociception, and hypothermia (Pertwee et al., 2007; Varvel et al., 2005). Twenty-four hours prior to the start of the experiment, mice were acclimated for 15 minute increments to the cold hotplate container and apparatus. On the experimental day, mice were brought into the experimental room and allowed to acclimate to the room settings for 30 minutes and then to the locomotor chamber for 30 minutes. Baseline readings for antinociception both spinal (tail-flick) and supraspinal (hotplate), catalepsy, and hypothermia were evaluated pre-injection. Animals were injected with either the vehicle (ethanol:cremophor:saline in a 1:1:18 ratio) or the test compound (10 mg/kg – 100 mg/kg). Animals were then allowed to individually quantify in the locomotor chamber (San Diego Instruments) for 30 minutes (expressed as the number of photo-beam breaks). The last 10 minutes of quantifying time was used for data analysis. Hotplate and tail-flick latencies (using cut off time of 45 seconds and 15 seconds, respectively, to reduce the chance of tissue damage), the change in core body temperature (°C) and catalepsy (seconds) were recorded at 30 minutes post-injection.
2.5.3 Data analysis
Data was presented as mean ± SEM with each group having n = 10 animals. Both hotplate and tail-flick were expressed as percent maximum effect (%MPE=[(post-drug latency-basal latency)/(cutoff latency-basal latency)] × 100 (Martin et. al., 1994). Statistical analysis was performed using one way ANOVA preceded by the Dunnett’s post hoc test to define significant difference against the vehicle control at p<0.05.
3. Results and discussion
A bioassay guided acid-base partitioning of the white and pink flower of N. nucifera EtOH extracts displayed in vitro κ- (91 % and 95%) and μ- (91 % and 96%) opioid receptor displacement activities for the basic CHCl3 partitions, while the acidic partitions were found to be inactive. UHPLC/APCI-MS analysis of basic partitions revealed seven major and three minor peaks (Table 1; SI). Masses of the major peaks were matched with nuciferine (1), N-nornuciferine (2), asimilobine (3), armepavine (4), O-methylcoclaurine (5), N-methylcoclaurine (6), coclaurine (7), N-methylasimilobine (8), and O-methylasimilobine (9) (Table 1), previously isolated from seeds and leaves of N. nucifera (Mukherjee et al., 2009; Nakamura et al., 2013) (Table 1). The masses of the two minor peaks appeared at [M+H]+ at m/z 611.2 and 625.7 were identified as dimeric bis-BTIQ alkaloids liensinine or isoliensinine and neferine (10), all were previously reported from embryos of N. nucifera (Itoh et al., 2011; Liu et al., 2009; Nakajima et al., 2007), while the third peak with [M+H]+ at m/z 623.4 could not be identified. In addition, compounds 1, 2, 8, and 9 were also identified from the acidic fractions by UHPLC/APCI-MS, together with two fatty acids [linoleic acid and palmitic acid], and fatty alcohol by GC/MS.
Table 1.
Preliminary identification of benzylisoquinoline alkaloids by UHPLC/ APCI-MS analysis
| Fraction | Rt (min) |
APCI-MS [M+H]+ |
Molecular weight |
Molecular formula |
Compounds identified |
|---|---|---|---|---|---|
| Basic Fraction | 3.727 | 286.2 | 285.34 | C17H19NO3 | Coclaurine (7) |
| 4.309 | 611.2 | 610.75 | C27H42N2O6 | Dimer (liensinine/isoliensinine) | |
| 4.589 | 314.2 | 313.39 | C19H23NO3 | Armepavine (4) | |
| 4.900 | 300.2 | 299.36 | C18H21NO3 | O-Methylcoclaurine (5) | |
| 5.106 | 300.4 625.7 |
299.36 624.8 |
C18H21NO3 C34H44N2O6 |
N-Methylcoclaurine (6) Neferine (10) |
|
| 5.370 | 282.2 | 281.34 | C18H19NO2 | N-Methylasimilobine (8) or 9 | |
| 5.612 | 268.2 | 267.3 | C17H17NO2 | Asimilobine (3) | |
| 5.623 | 282.2 | 281.34 | C19H23NO2 | O-Methylasimilobine (9) or 8 | |
| 6.471 | 282.2 | 281.34 | C19H23NO2 | N-nor-Nuciferine (2) | |
| 7.174 | 623.4 | - | - | Dimer, not identified | |
| Acidic Fraction | 7.792 | 296.2 | 295.38 | C19H21NO2 | Nuciferine (1) |
| 7.544 | 282.2 | 281.35 | C18H19NO2 | N-nor-Nuciferine (2) | |
| 5.668 | 282.2 | 281.35 | C18H21NO3 | N-Methylasimilobine (8) or 9 | |
| 4.931 | 282.2 | 281.35 | C18H21NO3 | O-Methylasimilobine (9) or 8 |
Fractionation of the basic CHCl3 partition of white flower, using CPTLC over customized alumina Chromatorotor™ (Muhammad et al., 2013), afforded compounds 2–7. Similarly, the acidic partition yielded compound 1 and additional quantities of 2. On the other hand, LA, mixture of LA and PA, and stigmasterol were isolated from acidic partition by CPTLC over silica gel. The structures of nuciferine (1), N-nor-nuciferine (2), asimiboline (3), armepavine (4), O-methylcoclaurine (5), and coclaurine (7) were determined by physical and full spectroscopic data, and also by comparison of their NMR data with those published in the literatures (Hong et al., 2010; Nieto et al., 1976; Rollinger et al., 2006; Tanahashi et al., 2006; Zhenjia et al., 2010; Asencio, et al., 1993) (Fig. 1). The 1H and 13C NMR data of (−)-N-methylcoclaurine (6), not fully reported previously, were assigned by comparing with spectral data of 4–7. As compound 10 was identified by UHPLC/APCI-MS (vide supra) and also detected in minute amounts in the basic partition, it was confirmed by comparing with an authentic sample of neferine (Rf 0.36, solvent: DCM:MeOH, 9:1; [α]D23.5 −19.99 (c 0.1, CHCl3, Lit. [α]D −37.8; (Furukawa, 1965); UHPLC/APCI-MS: [M+H]+ m/z 625.7 [M+H]+ C34H44N2O6+H), obtained from commercial sources. In addition, during the course of structural and bioactivity analysis of isolated BTIQs, two additional methylated analogs 12 and 13 were prepared, using trimethylsilyldiazomethane, from (±)-higenamine (11) (Hong et al., 2010), a constituent of N. nucifera which was obtained from commercial source. The specific rotation values and the NMR spectra of 11–13 supported the unambiguous assignments of 1H and 13C data of the isolated BTIQs and analogs. Finally, the identity of LA and PA was confirmed by GC/MS and by direct comparison with authentic commercial samples.
Fig. 1.
Structure of alkaloids and their analogs from N. nucifera
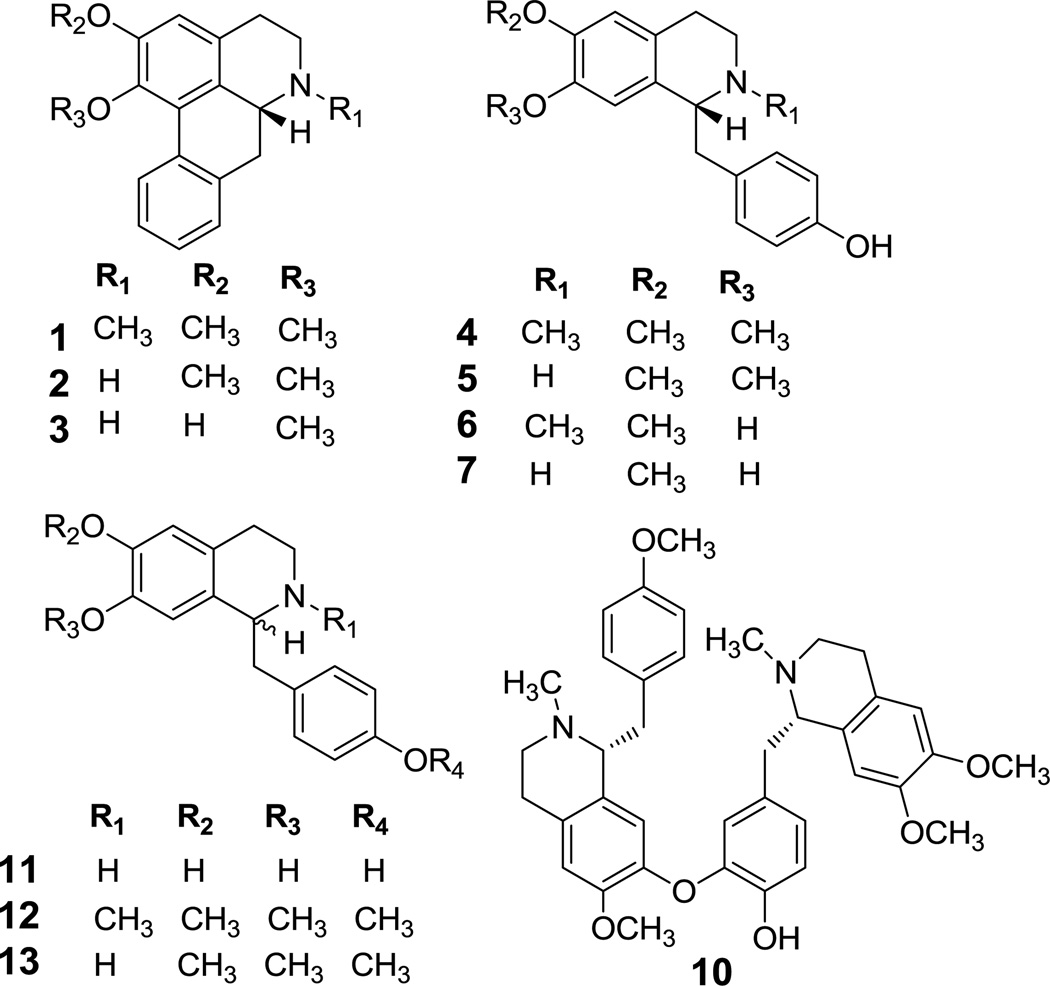
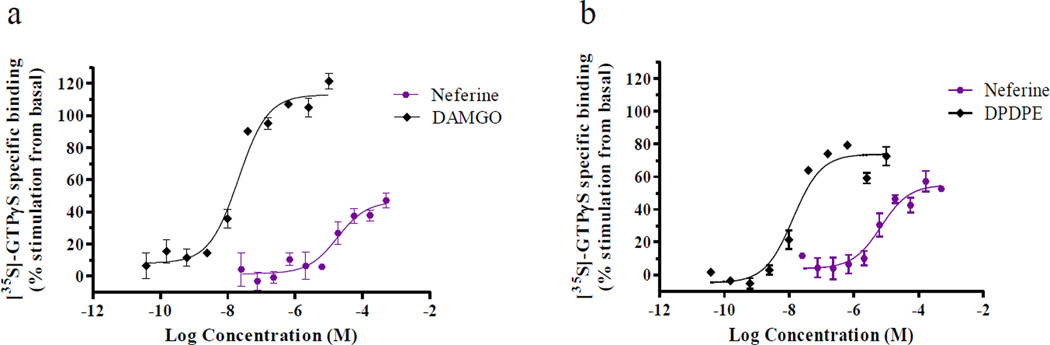
The EtOH extract, CHCl3 partitions, and pure compounds (1–13) were subjected to in vitro CB and opioid receptor radoligand displacement assays. The screening process showed that these extracts and compounds had no affinity for the cannabinoid receptor-1 or -2. Opioid IC50 and Ki values were determined for compounds that showed > ~50% displacement against opioid receptors, and GTPγS functional assays were also performed for compounds showing good binding affinities (Ki) to differentiate the ligands as agonist, partial agonist, antagonist, or inverse agonist. The basic CHCl3 partition and compounds 4–7 and 10 showed strong displacement of κ-and μ-opioid receptors (Table 2), whereas the acidic partition was found to be inactive. Based on primary displacement data, the Ki values of compounds 4–7 and 10 were determined (Table 3, Fig. 2). Among these, N-methylcoclaurine (6) showed prominent displacement towards κ opioid with Ki values of 0.9±0.1 µM, followed by coclaurine (7) and O-methylcoclaurine (5) (Ki 2.2±0.2 and 3.5±0.3 µM) (Fig. 2). Nevertheless, compounds (±)-11, (±)-12, and (±)-13, structural analogs of 6, did not show any significant displacements for opioid receptors, suggesting that racemization may diminish opioid displacement activity. In contrast, (−)-N-methylcoclaurine-derived dimer (−)-neferine (10) displayed strong displacement affinities for δ- and μ- opioid receptors with Ki values of 0.7±0.1 and 1.8±0.2 µM, respectively. Based on the GTPγS functional assay, 10 was determined to be a weak δ agonist (EC50 7.9±2.1 µM vs. 21.5±4.2 nM DPDPE) and weak μ partial agonist (EC50 >21 µM vs. 19.0±3.2 nM DAMGO) (Fig. 3).
Table 2.
Cannabinoid and opioid receptor displacements of extract, partitions, and compounds of N. nucifera flowers.
| Extracts/ partitions/ compounds | CB receptor displacement (%) (10 µg/mL) |
Opioid receptor displacement (%) (10 µg/mL) |
|||
|---|---|---|---|---|---|
| CB1 | CB2 | δ | κ | μ | |
| N. nucifera (white flower) EtOH extract | 17 | 14 | 13 | 46 | - |
| N. nucifera (pink flower) EtOH extract | 4 | 7 | 25 | 54 | - |
| N. nucifera (white flower) basic CHCl3 partition | - | 5 | 71 | 91 | 91 |
| N. nucifera (pink flower) basic CHCl3 partition | 26 | 26 | 53 | 95 | 96 |
| N. nucifera (white flower) acidic CHCl3 partition | 2 | 12 | 14 | 18 | 20 |
| N. nucifera (pink flower) acidic CHCl3 partition | 37 | 34 | 18 | 15 | 26 |
| Fraction G (PA:LA; 63.9:36.1)a | 6 | 12 | 91 | 51 | 87 |
| Fraction H (PA:LA; 89.5:10.5)a | 17 | - | 64 | 19 | 24 |
| Armepavine (4) | - | 14 | 46 | 66 | 65 |
| O-Methylcoclaurine (5) | - | 30 | 49 | 83 | 85 |
| N-Methylcoclaurine (6) | - | 13 | 55 | 92 | 83 |
| Coclaurine (7) | 15 | 8 | 51 | 91 | 83 |
| Neferine (10)b | - | - | 88 | 91 | 93 |
| Linoleic acid (LA)b | 30 | 14 | 60 | 19 | 34 |
| Palmitic acid (PA)b | 16 | 19 | 57 | 9 | 16 |
| LA + PA (1:1 mixture) | 4 | 7 | 96 | 50 | 69 |
| CP-55,940b | 96 | 100 | -c | -c | -c |
| Naloxone HClb | -c | -c | 100 | 100 | 101 |
Palmitic and linoleic acid mixture analyzed by GC/MS;
Tested at 10 µM,
Not applicable,
-: Not active
Table 3.
Ki values of compounds 4–7, 10, linoleic acid, and palmitic acid, for opioid receptors.
| Compound | δ Kia (µM) ± SEM | κ Ki a (µM) ± SEM | μ Ki a (µM) ± SEM |
|---|---|---|---|
| Armepavine (4) | 23.8 ± 3.7 | 13.0 ± 1.4 | 2.7 ± 0.8 |
| O-Methylcoclaurine (5) | 22.6 ± 3.9 | 3.5 ± 0.3 | 2.0 ± 0.3 |
| N-Methylcoclaurine (6) | 20.1 ± 3.1 | 0.9 ± 0.1 | 4.0 ± 0.5 |
| Coclaurine (7) | 21.1 ± 2.0 | 2.2 ± 0.2 | 5.0 ± 0.7 |
| Neferine (10) | 0.7 ± 0.1 | 3.3 ± 0.4 | 1.8 ± 0.2 |
| Linoleic acid (LA) | 14.5 ± 2.4 | -c | -c |
| Palmitic acid (PA) | >21.1 | -c | -c |
| LA + PA (1:1) | 9.2 ± 1.1 | -c | -c |
| U 69,593b | -c | 0.00099 ± 0.00093 | -c |
| DAMGOb | -c | -c | 0.0033 ± 0.0004 |
| Naloxone HClb | 0.0216 ± 0.0004 | 0.0021 ± 0.0002 | 0.0033 ± 0.0003 |
Ki: Equilibrium dissociation constant, a measure of the compounds binding affinity;
Positive controls;
not applicable.
Fig. 2.
Equilibrium constant (Ki) of compounds: (a) N-methylcoclaurine (6), O-methylcoclaurine (5), and coclaurine (7) against κ opioid receptor, (b) neferine (10) against μ opioid receptor, (c) neferine (10) against δ opioid receptor, Naloxone HCl was used as the positive control.
Fig. 3.
GTPγS functional assay of neferine (10): (a) μ-opioid (b) δ-opioid; DAMGO and DPDPE were used as the positive controls for μ- and δ-opioid receptors, respectively.
Fractionation of acidic partition also showed displacement of δ- and μ- receptors in Fr-G and Fr-H (Table 2), both were enriched with mixture of LA and PA in ratios of 64:36 and 89:11, respectively, analyzed by GC/MS. Nevertheless, a 1:1 mixture of pure LA and PA displayed an increase in displacement of δ receptor, compared to LA and PA when tested separately (Ki 9.2 for LA + PA vs. 14.5±2.4 for LA and >21.1 µM for PA). This observation was in agreement with those reported previously, suggesting that both acids have the ability to change the receptor conformation, thereby decreasing the availability of opioid binding sites (Tarawneh et al., 2013; Ingkaninan et al., 1999). These two fatty acids, previously isolated from N. nucifera (Zhao et al., 2013), appears to affect the over all in vitro opioid displacement activity of the extract perhaps by disrupting membrane stability.
N. nucifera acidic and basic partitions, compounds 1, 7, and mixture of 5–7 were tested for behavioral effects using in vivo mouse tetrad assay. This assay consist of four well established behavioral parameters, which is an indicator of classical cannabimimetic activity by inducing hypomotility, catalepsy, hypothermia, and analgesia, manifested by Δ9-THC and other cannabinoids (Martin et al., 1991; Pertwee et al., 2007). At a dose of 75–100 mg/kg/ip, the basic partition enriched with BTIQ alkaloids displayed decreased locomotion, and increased in antinociception, and hypothermia, while mixture of 5–7, isolated from basic partition, showed decreased locomotion and increased hypothermia at lower doses of the tetrad assay (Table 4). Coclaurine (7), the major alkaloid of the basic partition, did not influence any significant behavioral effects with the exception of weak locomotor activity, compared to those of basic partition and a mixture of 5–7. On the other hand, the acidic partition showed effects on all four parameters, inducing hypomotility, catalepsy, hypothermia, and analgesia, at a dose of 75–100 mg/kg/ip, which can be considered as weak cannabimimetic-type behavioral effect.
Table 4.
Behavioral effects of N. nucifera partitions, compounds 1, 7, and 5–7 using tetrad assay.
| Treatment (mg/Kg) |
Locomotor Activity |
Hotplate Latency (% MPE) |
Catalepsy Latency (Sec) |
Rectal temperature (°C) |
Tail-Flick Latency (% MPE)a |
|---|---|---|---|---|---|
| Basic CHCl3 partition | |||||
| 10 | 1538 ± 186.1 | 7.39 ± 4.08 | 3.13 ± 1.59 | −0.19 ± 0.08 | 9.44 ± 5.37 |
| 30 | 606.4 ± 127.0*** | 5.90 ± 4.87 | 1.0 ± 0.0 | −0.75 ± 0.19 | 15.35 ± 12.79 |
| 75 | 109.0 ± 109.0*** | 57.64 ± 11.80** | 16.38 ± 8.39 | −5.33 ± 0.44*** | 46.79 ± 13.84* |
| 100 | 69.25 ± 19.09*** | 74.05 ± 13.70*** | 13.75 ± 10.16 | −4.61 ± 0.31*** | 62.43 ± 15.53*** |
| Acidic CHCl3 partition | |||||
| 10 | 1315 ± 225.8 | 28.33 ± 6.48 | 1.50 ± 0.34 | −1.81 ± 0.43*** | 11.80 ± 10.49 |
| 30 | 165.3± 67.56*** | 9.44 ± 4.84 | 1.90 ± 0.41 | 0.11 ± 0.32 | 14.61 ± 7.819 |
| 75 | 91.3 ± 37.56*** | 89.46 ± 9.25*** | 10.20 ± 4.33 | −5.96 ± 0.24*** | 54.73 ± 14.99** |
| 100 b | 103.0 ± 33.81*** | 85.73 ± 9.19 *** | 71.60 ± 17.21*** | −7.2 ± 0.23*** | 70.05 ± 12.40*** |
| 5 – 7 mixture | |||||
| 10 | 1789 ± 163.9 | 10.58 ± 1.68 | 4.30 ± 1.43 | 0.50 ± 0.13 | 5.46 ± 4.72 |
| 30 | 1865 ± 131.0 | 13.33 ± 3.99 | 3.80 ± 1.63 | 0.62 ± 0.23 | −3.39 ± 6.89 |
| 75 | 137.8 ± 43.35*** | 14.83 ± 5.96 | 8.00 ± 3.81 | −2.49 ± 0.28*** | 22.09 ± 13.52 |
| 100 | 30.2 ± 7.318*** | 26.3 ± 6.57 | 7.89 ± 2.37 | −4.45 ± 0.24*** | 42.90 ± 15.68* |
| Coclaurine (7) | |||||
| 10 | 1678 ± 270.3 | 10.15 ± 4.45 | 1.25 ± 0.25 | 0.17 ± 0.08 | 16.57 ± 7.23 |
| 30 | 1798 ± 170.5 | 5.46 ± 5.05 | 2.37 ± 0.59* | 0.1 ± 0.15 | 1.43 ± 6.95 |
| 75 | 1132 ± 132.7 | 15.49 ± 3.57 | 1.75 ± 0.41 | −0.39 ± 0.05 | 13.95 ± 5.01 |
| 100 | 764.9 ± 194.0** | 6.08 ± 1.9 | 1.25 ± 0.25 | −0.58 ± 0.23* | 10.07 ± 3.31 |
| Nuciferine (1) | |||||
| 10 | 1318 ± 252.6 | 6.99 ± 2.61 | 3.875 ± 2.32 | −0.49 ± 0.31 | 11.69 ± 6.074 |
| 30 | 1022 ± 224.1 | 3.69 ± 3.01 | 1.750 ± 0.62 | −0.91 ± 0.31 | 18.43 ± 10.50 |
| 75 | 593.9 ± 268.2** | 0.68 ± 2.73 | 3.500 ± 1.07 | −1.68 ± 0.38** | 19.45 ± 6.16 |
| 100 | 165.1 ± 58.19*** | 21.78 ± 7.16 | 1.500 ± 0.27 | −3.8 ± 0.57*** | 14.14 ± 15.52 |
| Vehicles | |||||
| ETOH:Cre mophor:Sa line (1:1:18) | 1668 ± 109.5 | 10.81 ± 4.93 | 1.100 ± 0.10 | −0.09 ± 0.08 | −2.97 ± 7.66 |
MPE; % of maximum effect.
Acidic partition exhibited clonic-tonic seizures upon touch at a dose of 100 mg/kg. The following asterisks indicates the significances,
p<0.05,
p<0.01,
p<0.001 versus the vehicle, Dunnett’s post hoc test.
In addition, at 100 mg/kg/ip dose, all the mice had clonic-tonic seizures upon touch with the administration of acidic partition. However, (−)-nuciferine (1), the main constituent of the acidic partition, when tested showed only decreased locomotion and hypothermia at the same dose, but failed to display catalepsy and antinociceptive activity.
4. Conclusion
Analysis of the EtOH extracts of both the white and pink flowers of N. nucifera revealed the presence of seven alkaloids (1–7), two fatty acids (LA and PA) and a sterol (stigmasterol), in addition to 10, a minor alkaloid (Table 1). Among these, neferine (10) displayed considerable affinities for δ- and μ-opioid receptors and determined as a weak δ- agonist and weak μ partial agonist by GTPγS functional assay, while O- and N-methylcoclaurine, and coclaurine (5–7) showed affinities for κ-opioid receptor. The relatively low anticipated exposures and the limited affinity of the opioid receptor ligands, along with the presence of different types of constituents of N. nucifera flower (i.e., alkaloids and fatty acids) makes it tenuous to predict psychotropic opioidergic effects. All the tested compounds showed in vitro opioid receptor displacement at rather high concentrations, however, it was not clear if these affinities would relate to agonist, partial agonist, or antagonist effects, compared to reference standards, except for compound 10. Therefore, this would temper any considerations of concern for tolerance or addiction.
Studies of in vivo mouse tetrad assay revealed that the acidic partition showed weak cannabimimetic- type behavioral effect by affecting all four behavioral points of tetrad assay by inducing hypomotility, catalepsy, hypothermia, and analgesia. In addition, the mice had clonic-tonic seizures upon touch. The acidic partition is enriched with aporphines 1 and 2, and fatty acids, PA and LA. Nuciferine (1), an analog of apomorphine, has been reported previously as neuroleptic (i.e., like chloropromazine) and dopamine blocker (Bhattacharya et al., 1978; Macko et al., 1972), and acetylcholinesterase inhibitor (Yang et al., 2014), though it failed to show any cataleptic effect or toxicity in the form of tremors and convulsions in the tetrad assay. Based on foregoing discussion, it can be concluded that the in vivo mild cannabimimetic-type effect observed for the aporphine-enriched CHCl3 partition, as well as decreased locomotion and increased antinociception and hypothermia of the BTIQ-enriched basic partition is likely mediated by other CNS mechanisms since the extracts, partitions, and isolated compounds from N. nucifera had no affinity for the in vitro CB1 or CB2 receptors. A possible mechanism of many BTIQ alkaloids has previously been attributed to dopamine and serotonergic receptors that controls neuropsychiatric disorders (Bhattacharya et al., 1978; Cabedo et al., 2009; Macko et al., 1972; Morais et al., 1998). The above data suggested that the behavioral effects displayed by the acidic CHCl3 partition could be due to a combined effect of aporphine alkaloids and/or other constituents present in the partition. This work, along with traditional use and the reported bioactivities of the BTIQ and aporphine alkaloids, suggested further studies on N. nucifera are needed to understand the roles that the extracts and/ or individual compounds might contribute to behavioral effects.
Supplementary Material
Acknowledgement
This study was supported by an Institutional Development Award (IDeA) Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS) and the Research Cores C and D of the COBRE, a component of the National Institutes of Health (NIH) under the grant number P20GM104932. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH. The authors would like to thank Dr. Larry A. Walker, School of Pharmacy, for valuable advice, Dr. J. Zhang for UHPLC-MS data analysis, Dr. Vijayasankar Raman for preparing vouchers of the plant material, and Dr. Mohamed A. Ibrahim for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asencio M, Cassels BK, Speisky H, Valenzuela A. (R)-and (S)-coclaurine from the bark of Peumus boldus. Fitotherapia. 1993;64:455–458. [Google Scholar]
- Bhattacharya SK, Bose R, Ghosh P, Tripathi VJ, Kay AB, Dasgupta B. Psychopharmacological studies on (−)-nuciferine and its Hofmann degradation product atherosperminine. Psychopharmacology. 1978;59:29–33. doi: 10.1007/BF00428026. [DOI] [PubMed] [Google Scholar]
- Buddhadev SG, Buddhadev SS. Nelumbo nucifera the phytochemical profile and traditional uses. Pharma Science Monitor. 2014;5:1–12. [Google Scholar]
- Cabedo N, Berenguer I, Figadere B, Cortes D. An overview on benzylisoquinoline derivatives with dopaminergic and serotonergic activities. Current Medicinal Chemistry. 2009;16:2441–2467. doi: 10.2174/092986709788682100. [DOI] [PubMed] [Google Scholar]
- Chiang Su New Medical College. “Zhong-yao-dai-ci-dian “Dictionary of Chinese crude drugs”. Vol. 1806. Shanghai: Shanghai Scientific Technologic Publisher; 1978. [Google Scholar]
- Duke JA, Bogenschutz-Godwin MJ, duCellier J, Duke AK. Hand Book of Medical Herbs 2002. Second ed. Boca Raton: CRC press; 2002. p. 473. [Google Scholar]
- Furukawa H. On the alkaloids of Nelumbo nucifera Gaertn. IX. Alkaloids of loti embryo (2). Structure of neferine, a new biscoclurine alkaloid. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan. 1965;85:335–338. [PubMed] [Google Scholar]
- Gao J, Radwan MM, León F, Dale OR, Husni AS, Wu Y, Lupien S, Wang X, Manly SP, Hill RA, Dugan FM, Cutler HG, Cutler SJ. Neocosmospora sp-derived resorcylic acid lactones with in vitro binding affinity for human opioid and cannabinoid receptors. Journal of Natural Products. 2013;76(5):824–828. doi: 10.1021/np300653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti RD, Duchek J, Werner L, Husni AS, McCurdy CR, Cutler SJ, Cox DP, Hudlicky T. Heteroatom analogues of hydrocodone: Synthesis and biological activity. The Journal of Organic Chemistry. 2013;78:2914–2925. doi: 10.1021/jo3026753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Lee Y-I, Jin D. Determination of (+)-higenamine enantiomer in Nelumbo nucifera by high-performance liquid chromatography with a fluorescent chiral tagging reagent. Microchemical Journal. 2010;96:374–379. [Google Scholar]
- Husni AS, McCurdy CR, Radwan MM, Ahmed SA, Slade D, Ross SA, ElSohly MA, Cutler SJ. Evaluation of phytocannabinoids from high-potency Cannabis sativa using in vitro bioassays to determine structure–activity relationships for cannabinoid receptor 1 and cannabinoid receptor 2. Medicinal Chemistry Research. 2014;23:4295–4300. doi: 10.1007/s00044-014-0972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingkaninan K, von Frijtag Drabbe Kunzel JK, IJzerman AP, Verpoorte R. Interference of linoleic acid fraction in some receptor binding assays. Journal of Natural Products. 1999;62:912–914. doi: 10.1021/np9805490. [DOI] [PubMed] [Google Scholar]
- Itoh A, Saitoh T, Tani K, Uchigaki M, Sugimoto Y, Yamada J, Nakajima H, Ohshiro H, Sun S, Tanahashi T. Bisbenzylisoquinoline alkaloids from Nelumbo nucifera. Chemical and Pharmaceutical Bulletin. 2011;59:947–951. doi: 10.1248/cpb.59.947. [DOI] [PubMed] [Google Scholar]
- Journigan VB, Mésangeau C, Vyas N, Eans SO, Cutler SJ, McLaughlin JP, Mollereau C, McCurdy CR. Nonpeptide small molecule agonist and antagonist original leads for neuropeptide FF1 and FF2 receptors. Journal of Medicinal Chemistry. 2014;57:8903–8927. doi: 10.1021/jm500989n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Shin D, Oh JW, Cho C, Lee HJ, Yoon DW, Bae H. The antidepressant effect of Nelumbinis semen on rats under chronic mild stress induced depression-like symptoms. The American Journal of Chinese Medicine. 2005;33(02):205–213. doi: 10.1142/S0192415X05002874. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y, Aoshima A, Ikeshiro Y, Chen Y-P, Furukawa H, Itoigawa M, Fujioka T, Mihashi K, Cosentino LM, Morris-Natschke SL, Lee KH. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorganic and Medicinal Chemistry. 2005;13:443–448. doi: 10.1016/j.bmc.2004.10.020. [DOI] [PubMed] [Google Scholar]
- León F, Gao J, Dale OR, Wu Y, Habib E, Husni AS, Hill RA, Cutler SJ. Secondary metabolites from Eupenicillium parvum and their in vitro binding affinity for human opioid and cannabinoid receptors. Planta Medica. 2013;79:1756–1761. doi: 10.1055/s-0033-1351099. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang B, Li XZ, Qi LF, Liang YZ. Preparative separation and purification of liensinine, isoliensinine and neferine from seed embryo of Nelumbo nucifera GAERTN using high speed counter-current chromatography. Journal of Separation Science. 2009;32:2476–2481. doi: 10.1002/jssc.200800766. [DOI] [PubMed] [Google Scholar]
- Macko E, Douglas B, Weisbach JA, Waltz DT. Studies on the pharmacology of nuciferine and related aporphines. Archives Internationales de Pharmacodynamie et de thérapie. 1972;197:261–273. [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacology Biochemistry and Behaviour. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Prescott WR, Barret RL, Razdan RK. Pharmacological evaluation of dimethyl heptyl analogs of Δ9-THC: reassessment of the putative three-point cannabinoid-receptor interaction. Drug and Alcohol Dependence. 1994;37:231–240. doi: 10.1016/0376-8716(94)01081-u. [DOI] [PubMed] [Google Scholar]
- Mehta NR, Ekta P, Pragnesh P, Patani V, Shah B. Nelumbo nucifera (lotus): a review on ethnobotany, phytochemistry and pharmacology. Indian Journal of Pharmaceutical and Biological Research. 2013;1(4):152–167. [Google Scholar]
- Mathew M, Subramanian S. In Vitro Screening for anticholinesterase and antioxidant activity of methanolic extracts of Ayurvedic medicinal plants used for cognitive disorders. PLoS ONE. 2014;9:1, e86804. doi: 10.1371/journal.pone.0086804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Kapoor LD. Kamala -The national flower of India - Its ancient history and uses in Indian medicine. Indian Journal of History of Science. 1976;11(2):125–132. [PubMed] [Google Scholar]
- Morais LC, Barbosa-Filho JM, Almeida RN. Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice. Journal of Ethnopharmacology. 1998;62:57–61. doi: 10.1016/s0378-8741(98)00044-0. [DOI] [PubMed] [Google Scholar]
- Muhammad I, Samoylenko V, Machumi F, Zaki M, Mohammed R, Hetta M, Gillum V. Preparation and application of reversed phase chromatorotor™ for the isolation of natural products by spin chromatographic system. Natural Product Communications. 2013;8:311–314. [PubMed] [Google Scholar]
- Mukherjee PK, Balasubramanian R, Saha K, Saha BP, Pal M. A review on Nelumbo nucifera gaertn. Ancient Science of Life. 1996;15:268–276. [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Saha K, Balasubramanian R, Pal M, Saha BP. Studies on psychopharmacological effects of Nelumbo nucifera Gaertn. Rhizome extract. Journal of Ethnopharmacology. 1996;54:63–67. doi: 10.1016/s0378-8741(96)01455-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera)– phytochemical and therapeutic profile. Journal of Pharmacy and Pharmacology. 2009;61:407–422. doi: 10.1211/jpp/61.04.0001. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Sugimoto Y, Sun S-J, Tanahashi T, Yamada J. Benzylisoquinoline derivative-or bisbenzylisoquinoline derivative-containing psychotropic agent, analgesic and/or antiphlogistic, and health food. US2007/0027181 A1. US patent number. 2007
- Nakamura S, Nakashima S, Tanabe G, Oda Y, Yokota N, Fujimoto K, Matsumoto T, Sakuma R, Ohta T, Ogawa K, Nishida S, Miki H, Matsuda H, Muraoka O, Yoshikawa M. Alkaloid constituents from flower buds and leaves of sacred lotus (Nelumbo nucifera, Nymphaeaceae) with melanogenesis inhibitory activity in B16 melanoma cells. Bioorganic and Medicinal Chemistry. 2013;21:779–787. doi: 10.1016/j.bmc.2012.11.038. [DOI] [PubMed] [Google Scholar]
- Nguyen Q. Lotus A new crop for Australian horticulture. IHD: Access to Asia Newsletter. 1999;(2):1–5. [Google Scholar]
- Nieto M, Sevenet T, Leboeuf M, Cave A. Alkaloids of Annonaceae: Alkaloids of Xylopia pancheri. Planta Medica. 1976;30:48–58. [PubMed] [Google Scholar]
- O’Mahony Carey S. Psychoactive substances: A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. [Accessed on October 11, 2014];2010 http://www.drugs.ie/resourcesfiles/guides/Psychoactive_substances_low_res.pdf. [Google Scholar]
- Orejarena Pacheco JC, Lahm G, Opatz T. Synthesis of alkaloids by Stevens rearrangement of nitrile-stabilized ammonium ylides:(±)-laudanosine,(±)-laudanidine,(±)-armepavine,(±)-7-methoxycryptopleurine, and (±)-xylopinine. The Journal of Organic Chemistry. 2013;78:4985–4992. doi: 10.1021/jo400659n. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, Martin BR, Razdan RK. The psychoactive plant cannabinoid, Δ9-tetrahydrocannabinol, is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. British Journal of Pharmacology. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield WM. The principle Chinese vegetable foods and food plants of China town. Economic Botany. 1951;5:10–12. [Google Scholar]
- Rollinger JM, Schuster D, Baier E, Ellmerer EP, Langer T, Stuppner H. Taspine: bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia x soulangiana. Journal of Natural Products. 2006;69:1341–1346. doi: 10.1021/np060268p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengkhae C, Arunnopparat W, Sungkhajorn P. Antioxidant activity of Nelumbo nucifera Gaertn on oxidative stress-induced Erythrocyte hemolysis in hypertensive and normotensive rats. Thai Journal of Physiological Sciences. 2008;20:70–78. [Google Scholar]
- Shoji N, Umeyama A, Saito N, Iuchi A, Takemoto T, Kajiwara A, Ohizumi Y. Asimilobine and Lirinidine, Serotonergic Receptor Antagonists, from Nelumbo nucifera. Journal of Natural Products. 1987;50:773–774. doi: 10.1021/np50052a044. [DOI] [PubMed] [Google Scholar]
- Tanahashi T, Yamada J, Nakajima H, Sun S-J. Method and health food for preventing and/or alleviating psychiatric disorder, and/or for effectuating sedation. US20060030586 A1. US patent number. 2006
- Tarawneh AH, León F, Radwan MM, Wang X, Dale OR, Husni AS, Rosa LH, Cutler SJ. Fatty acids with in vitro binding affinity for human opioid receptors from the fungus Emericella nidulans. Journal of Agricultural and Food Chemistry. 2013;61:10476–10480. doi: 10.1021/jf4019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta 9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;314(1):329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Velusami CC, Agarwal A, Mookambeswaran V. Effect of Nelumbo nucifera petal extracts on lipase, adipogenesis, adipolysis, and central receptors of obesity. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1–7. doi: 10.1155/2013/145925. Article ID #145925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Song Z, Xue W, Sheng J, Shu Z, Shi Y, Liang J, Yao X. Synthesis and structure–activity relationship of nuciferine derivatives as potential acetylcholinesterase inhibitors. Medicinal Chemistry Research. 2014;23:3178–3186. [Google Scholar]
- Zhao X, Shen J, Chang KJ, Kim SH. Analysis of fatty acids and phytosterols in ethanol extracts of Nelumbo nucifera seeds and rhizomes by GC-MS. Journal of Agricultural and Food Chemistry. 2013;61:6841–6847. doi: 10.1021/jf401710h. [DOI] [PubMed] [Google Scholar]
- Zhenjia Z, Minglin W, Daijie W, Wenjuan D, Xiao W, Chengchao Z. Preparative separation of alkaloids from Nelumbo nucifera leaves by conventional and pH-zone-refining counter-current chromatography. Journal of Chromatography B. 2010;878:1647–1651. doi: 10.1016/j.jchromb.2010.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.