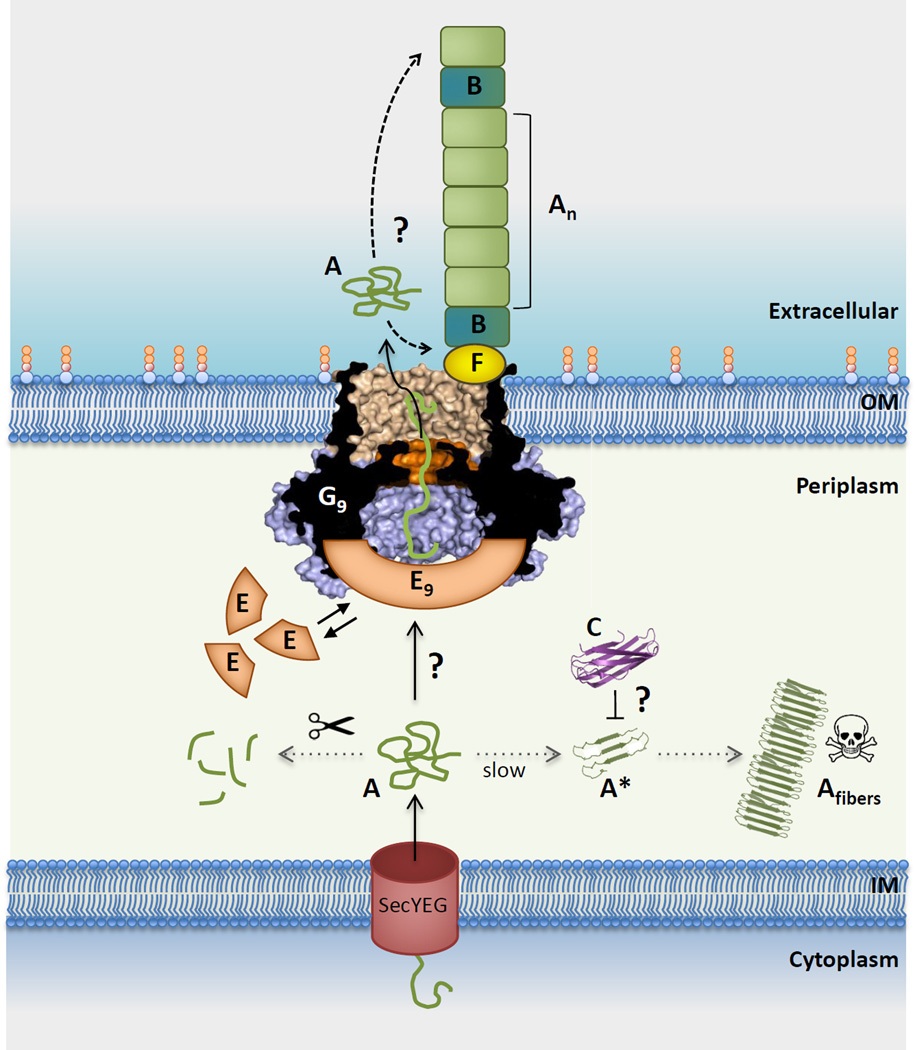
Figure 5. Integrated model for curli subunit secretion.
CsgA (A) subunits enter the periplasm via the SecYEG translocon, from where they progress to the cell surface via the curli transporter CsgG (G) or are proteolytically degraded (left dotted line). Premature polymerization of CsgA in the periplasm (right dotted line) is subverted by CsgC (C), probably by the binding and neutralization of early assembly intermediates (labeled A*). CsgG forms a nonameric complex (G9) that acts as a non-specific peptide diffusion channel. A nonameric CsgE complex (E9) acts as specificity and secretion factor to the CsgG channel and forms a capping structure to a pre-constriction cavity in the CsgG complex. Recruitment and (partial) enclosure of CsgA in the pre-constriction cavity is proposed to create an entropy gradient over the channel that favors CsgA’s outward diffusion as an unfolded, soluble polypeptide. Once secreted, curli fiber formation and elongation is templated by CsgB (B), in a CsgF (F)-dependent manner. It is unknown whether fibers grow from the proximal or distal end (dashed arrows). Also, the sequence that leads to the assembly of the CsgF:CsgG:CsgE:CsgA secretion complex, or its stoichiometry (i.e. G9:E9 + n CsgA subunits) are currently unknown [23]), with three possible scenarios as follows: (1) does an encounter of CsgE and CsgA in the periplasm lead to their docking onto CsgG?; (2) or does CsgG itself recruit CsgA prior to the binding of the capping CsgE nonamer?; (3) or are CsgG and CsgE in a constitutive complex that is dynamically gated in function of CsgA binding? Further experimental and structural work is needed to resolve these mechanistic questions in the curli secretion pathway. Abbreviations: IM, inner membrane; OM, outer membrane.