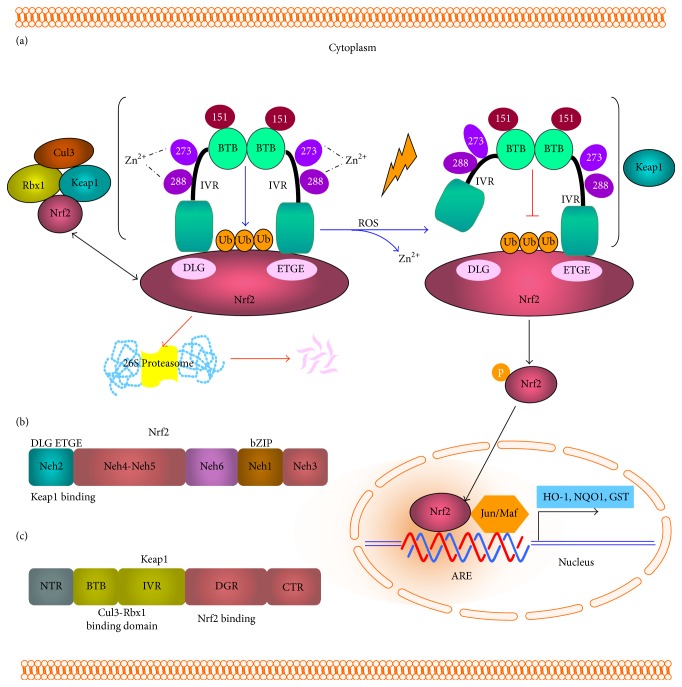
Figure 2.
The Kelch-like chicken erythroid-derived cap “n” collar homology factor-associated protein 1- (Keap1-) NF-E2-related factor 2- (Nrf2) antioxidant response element (ARE) signaling pathway. (a) Keap1-Nrf2 interactions are mediated via the high affinity ETGE motif “hinge” site and the lower affinity DLG motif “latch” site within the Nrf2 Neh2 domain. Under normal cellular conditions, Nrf2 first interacts with the Keap1 dimer through the ETGE hinge and subsequently with the cullin-3- (Cul3-) ring box 1 (Rbx1) complex via the DLG latch motif, which leads to the ubiquitination and degradation of Nrf2. During cellular stress, the hinge and the latch of Nrf2 may be disrupted by changes to Keap1 homodimer formation and translocation to nucleus. (b) The structure of Nrf2, including Neh1–6 domains. (c) Keap1 is composed of distinct structural domains, including the N terminal region (NTR), Broad complex, Tramtrack, and bric-a-brac (BTB) domain, intervening region (IVR), double glycine repeat (DGR; Nrf2 binding region), and C terminal region (CTR).