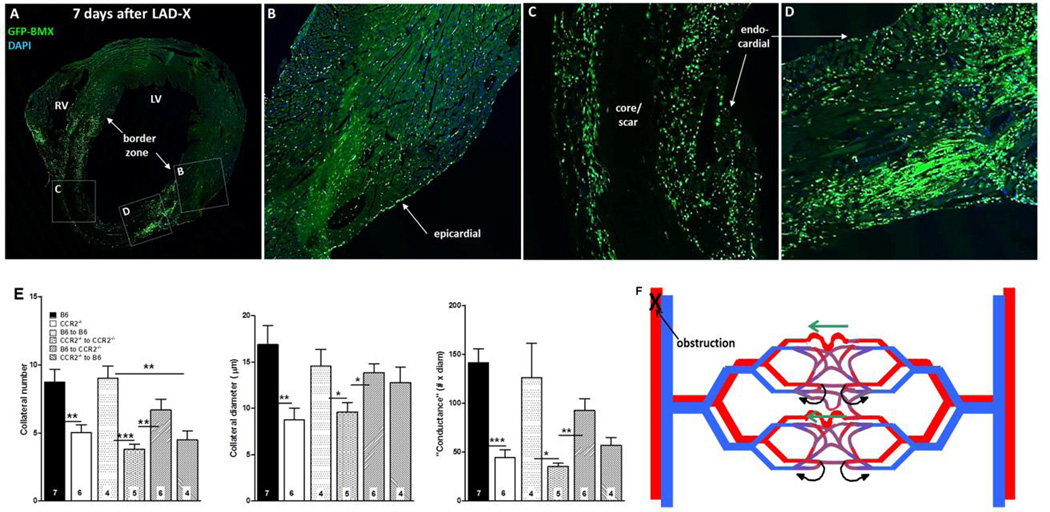
Figure 7.
A–D, Bone-marrow–derived cells home to myocardium. Wildtype B6 mouse 7 weeks after bone-marrow (BM) transplant with B6-GFP-expressing BM cells, and 7 days after LAD ligation. Confocal images (A, coronal section of heart at low magnification) show high number of cells in border zone (A,D), intermediate number surrounding core/scar region (A,C), low number in remote zone (A,B) and very low number in core/scar (A,C). BM cells also evident in epicardial and endocardial surface layers. E, Transplant of wildtype BM cells rescues deficient neo-collateral formation, and transplant of CCR2−/− BM cells inhibits normal neo-collateral formation in wildtype (B6) and CCR2−/− mice 1 week after LAD ligation. Methods are same as in Figure 2. Controls (bars 3 and 4 in each graph) show no effect of the transplant procedure. *, **, *** p<0.05, 0.01, 0.001. Figure 7F. Model of neo-collateral formation. Blood flow in the capillary plexus in watershed areas between trees normally returns via contiguous venous trees (curved black arrows), consistent with Evans blue perfusion data shown in Online Figures 3–5. Arterial obstruction causes ischemia and increased fluid shear stress, the latter especially in the largest-diameter capillary(s). MCP1, fractalkine and presumably other factors activate CCR2, CX3CR1 and other proteins, leading to recruitment of immune cells and vascular and/or hematopoietic progenitors from bone marrow and possibly additional sites. The resulting milieu of “remodeling” and growth factors directs pruning of venous connections from the largest capillaries over day 0–1, followed by increase in diameter, length and mural cell recruitment over day 1–7.