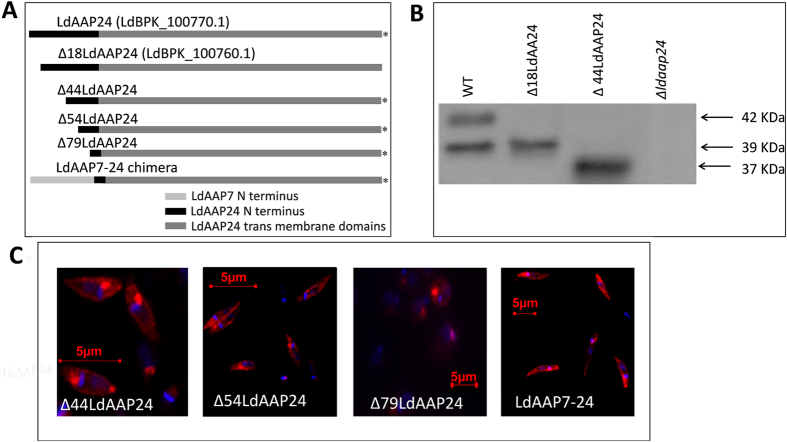
Figure 2. Truncating the hydrophilic N-terminus of LdAAP24 does not affect membrane localization.
(A) Schematic representation of the different LdAAP24 variants and the length of the N-terminus in each construct. LdAAP24 is the full length variant with an N-terminus of 89 aa; Δ18LdAAP24 has an N-terminus of 71 aa; Δ44LdAAP24 has an N-terminus of 45 aa; Δ54LdAAP24 has an N-terminus of 35 aa; Δ79LdAAP24 has an N-terminus of 10 aa; and LdAAP7-24 has a chimeric N-terminus of 94 aa. Constructs marked with (*) were HA-tagged at the C-terminus. (B) Proteins were extracted from the following promastigote strains: WT, LdAAP24-null mutants ectopically expressing Δ18LdAAP24, LdAAP24-null mutants ectopically expressing Δ44LdAAP24, or LdAAP24-null mutant promastigotes (Δldaap24). Western blot analysis was performed using anti-LdAAP24 antibody. (C) Indirect immunofluorescence of LdAAP24 in LdAAP24-null mutants (Δldaap24) ectopically expressing Δ44LdAAP24, Δ54LdAAP24, Δ79LdAAP24 or LdAAP7-24 chimera. Cells were stained with anti-LdAAP24 or with anti-HA antibodies (red) and the DNA stained with DAPI (blue), the latter stains the nucleus and the kinetoplast. The two fluorescent images were merged.