Figure 3. Truncating the hydrophilic N-terminus of LdAAP24 affects substrate translocation activity.
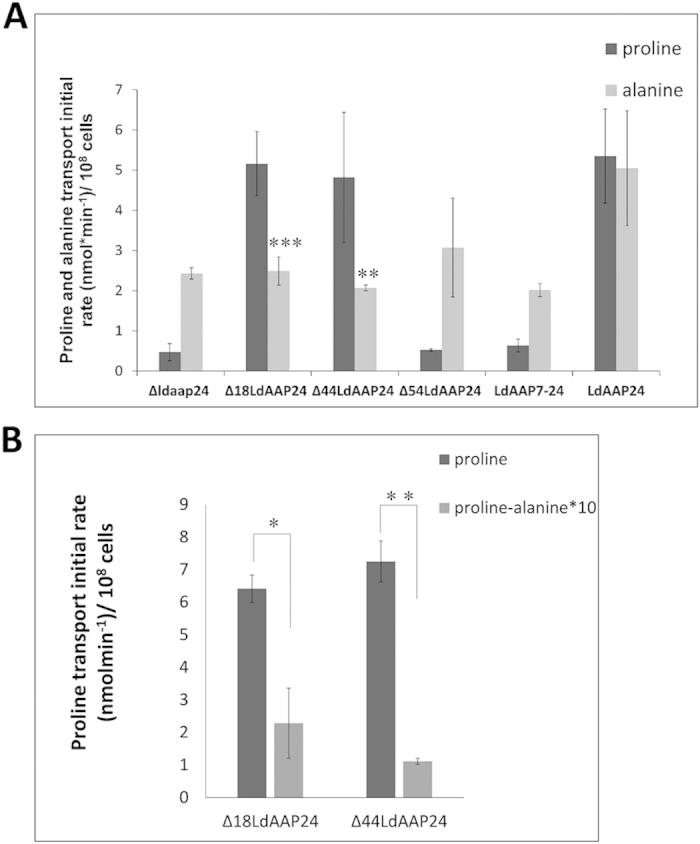
(A) Initial transport rate (3 minutes) of 1 mM 3H L-proline (dark grey) and 1 mM 3H L-alanine (light grey) were assayed in the following strains: LdAAP24-null mutants (Δldaap24), Δldaap24 ectopically expressing Δ18LdAAP24, Δldaap24 ectopically expressing Δ44LdAAP24, Δldaap24 ectopically expressing Δ54LdAAP24, Δldaap24 ectopically expressing LdAAP7-24 chimera and Δldaap24 ectopically expressing full length LdAAP24. Transport was determined at pH 7 and 30 °C. Values represent the mean of at least three independent repeats ± SD. Statistically significant differences (two-tailed T-test) in alanine transport relative to full-length LdAAP24 (p ≤ 0.005) are marked with (**) and (p ≤ 0.001) are marked with (***) (B) Initial transport rate (3 minutes) of 1 mM 3H L-proline (dark grey) and 3H L-proline in the presence of 10-fold concentrations of alanine (light grey) were assayed in Δldaap24 ectopically expressing Δ18LdAAP24 or Δ44LdAAP24. Transport was determined at pH 7 and 30 °C. Values represent the mean of at least three independent repeats ± SD. Statistically significant differences (p ≤ 0.05) are marked with (*) and (p ≤ 0.005) are marked with (**).
