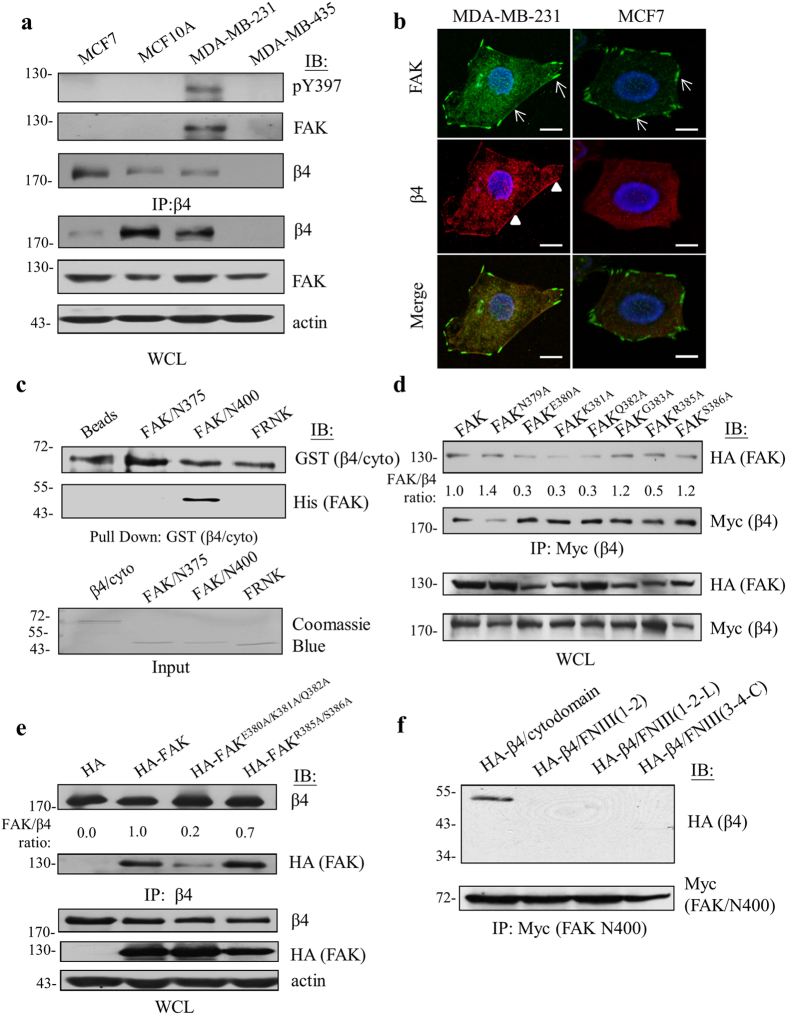
Figure 1. The physical interaction of β4 integrin and FAK is associated with tumor malignancy in vivo and in vitro.
(a) Varied human cancer cell lines were analyzed by Western blot analysis with anti-β4 integrin, anti-FAK, or anti-phospho-Tyr397 antibody, showing an interaction between β4 integrin and FAK. The human mammary epithelial cell line MCF10A was used as a normal control. (b) MDA-MB-231 (aggressive) and MCF7 (non-aggressive) cells were stained to show the co-localization of FAK (green, arrows) and β4 integrin (red, arrowheads) on the peripheral plasma membrane in MDA-MD-231 cells but not in MCF7 cells. Scale bars, 10 μm. (c) The association between β4 integrin and FAK-derived recombinant proteins was determined by an in vitro binding assay. (d) By immunoprecipitation and Western blot analysis, the crucial amino acids that were responsible for interaction with β4 integrin were determined. The mean of the relative interaction between β4 integrin and FAK (normalized to wild-type FAK shown as 1.0) was measured. (e) The triple amino acids (FAKE380A/K381A/Q382A) exhibited a marked reduction in β4 integrin binding compared to wild-type FAK or the double (FAKR385A/S386A) mutant. (f) The cytodomain of β4 integrin is indispensable to its interaction with FAK. Each experiment was repeated at least three independent times and the densitometric analysis of the relative quantification of band intensities, normalized to respective controls, is shown in Supplementary Fig. S6. All cropped blots were run under the same experimental conditions. The full-length blots are included in Supplementary Fig. S7.