Abstract
Local myocardial application of inotropes may allow the study of pharmacologically augmented central myocardial contraction in the absence of confounding peripheral vasodilating effects and alterations in heart loading conditions. Novel alginate epicardial (EC) drug releasing platforms were used to deliver dobutamine to the left ventricle of rats. Pressure volume analyses indicated that while both local and systemic (IV) use of inotropic drugs increase stroke volume and contractility, systemic infusion does so through heart unloading. Conversely, EC application preserves heart load and systemic blood pressure. Epicardial dobutamine increased indices of contractility with less rise in heart rate and lower reduction in systemic vascular resistance than IV infusion. Drug sampling showed that dobutamine concentration was 650-fold higher in the anterior wall than in the inferior wall The plasma dobutamine concentration with local delivery was about half as much as with systemic infusion. These data suggest that inotropic EC delivery has a localized effect and augments myocardial contraction by different mechanisms than systemic infusion, with far fewer side effects. These studies demonstrate a pharmacologic paradigm that may improve heart function without interference from effects on the vasculature, alterations in heart loading and may ultimately improve the health of heart failure patients.
Keywords: Epicardial drug delivery, inotrope, myocardial contraction, dobutamine and heart failure
Introduction
Classic teaching holds that local delivery avoids the dosing inefficiency of systemic delivery. Intravascular infusion attains whole-body drug exposure rapidly and is ideal for circulating or systemic diseases, but problematic when drug needs to be delivered to specific organs or tissues. In this latter case, systemic administration delivers drug everywhere, reducing the net amount of administered drug that reaches the desired target along with an associated potential for systemic side effects and toxicities. Dose reduction can eliminate the toxic effects but reduces benefit as well. Local application to a site-specific organ or target has the potential to confine drug to the target tissues, potentially reducing cost, side effect and toxicity. The focus on the detriment of toxic dosing to undesired targets and beneficial effects of targeted administration has obscured other important issues related to local delivery. In particular emerging data suggest that the high levels of vascularization of some organs will lead to clearance of drug from the target and therefore significant systemic drug concentrations.1,2 The question then arises as to whether and why local delivery can demonstrate beneficial effects even at doses that lead to detectable drug in the periphery. Is it possible for example, that there is a fundamentally different pharmacologic effect or differential pharmacokinetics for systemic infusion and local delivery?
Inotropic therapies for heart failure offer a perfect example of the complexity of local delivery. The cardiovascular system involves organs of complex structure but also a linked system where effects on one organ element elicit reflex responses throughout the system. Changes in myocardial function induce reflex effects on the entire vasculature and alterations in vascular tone change the loading conditions on the heart. Thus, local cardiovascular drug delivery can affect organs at a distance through a direct contact effect as drug is rapidly transported from local site through the arterial system and indirectly as a result of rapid reflex responses to cardiac output, tissue perfusion and myocardial loading conditions. Moreover, the density and variety of receptors and target proteins for the same drug can vary widely. Adrenergic receptor subtype differs in the heart (β1) and blood vessels (β2) and response to the same stimuli is mediated through different pathways in the myocardium (Stimulatory G proteins) and vasculature (Inhibitory and Stimulatory G proteins).3-5 Systemic toxicity is indeed reduced with reduction of systemic dose but there might well be a benefit to local delivery that transcends reduction in toxicity.
We postulate therefore that the local administration of drugs that affect myocardial contraction will not only enhance the therapeutic window for these drugs but also potentially allow them to act in a more selective manner. Local delivery might not only reduce toxicity from high doses but induce physiologic responses that are distinctly different than systemic infusion. We created a model system which divorced the central contractile effects of inotropic drug therapy from the arterial-dilating peripheral effects using a local polymeric controlled epicardial (EC) release preparation which delivered drug to the outer surface of the heart. Bi-concave calcium-crosslinked alginate discs released dobutamine to the heart surface with zero-order kinetics over a wide range of controlled rates. These alginate drug delivery devices allowed us to compare the dose response of local epicardial dobutamine application with that of intravenous infusion (IV), in terms of indices of contractility and peripheral side effect, which were assessed using Millar left ventricular pressure-volume conductance catheters in healthy adult rats.6,7 These experiments allowed us to study the impact of drugs on the heart itself, without the confounding effects of altered loading conditions.
Materials and Methods
Fabrication and in vitro characterization of EC inotrope delivery platform
A novel system for precisely controlling the rate of dobutamine release to the epicardial (EC) surface of the heart over a wide range of doses allowed characterization of the dose response for comparison to IV infusion. Epicardial drug releasing platforms were constructed from calcium crosslinked alginate hydrogels8-10 and served to apply drug over the anterior surface of the rat heart. Alginate (Sigma-Aldrich #71238, St.Louis, MO) discs were made by crosslinking 45 μl of a 2% slurry in double distilled water (ddH20) at room temperature in the upper chamber of a transwell permeable co-culture support (6.5mm, polyester, 3 μm pore size, Corning #3472). The transwell support was immersed in 1 ml of 3% CaCl2 in ddH20 for 1.5 hours using a leveled 24 well culture plate (Corning #353047, 15.75 mm). The meniscus in the original alginate slurry along the walls of the transwell support was preserved during calcium crosslinking so the resulting concave disc had a minimal thickness at the center of 0.6 mm and a maximal thickness along the edge of 1.4 mm. The alginate slabs were removed from the transwell support by carefully cutting away the polyester membrane. Drug added in liquid solution to the top of the concave alginate disc diffuses across and is released out the bottom. The calcium was removed from the concave discs prior to use in vitro or in vivo by soaking in 40 ml of ddH20 for 1.5 hour.
Preliminary experiments showed that starting with 1.5% alginate yielded flimsy hydrogels that lacked mechanical integrity while 2.5 and 3% alginate produced hydrogels that were too rigid to conform to the heart surface. Other preliminary experiments showed that the 6.5 mm diameter produced devices that fit the heart surface better than 12mm diameter hydrogels. The final weight of the devices were minimized to allow it to stay on the heart while beating, yet provided a reasonable minimal thickness in the center of the concavity to provide mechanical resilience and prevent an initial burst release of drug.
The in vitro release of dobutamine from calcium crosslinked concave alginate discs was characterized (Fig. 1a). The alginate disc was placed into a new transwell support which was immersed in ddH20 (340 μl) in the lower chamber of a 24 well culture plate. The culture plate was placed on a rocker platform (20 RPM). In separate experiments, a 5 μl volume of dobutamine (1.39, 2.08, 3.125, 4.69, 6.25, 9.38, or 12.5 mg/ml in ddH20, Hospira, Lake Forest, Il) was placed on the upper surface of the alginate disc. Samples for drug concentration measurements (60 μl) were withdrawn at 3 time points. These relatively large volumes were needed to meet the sensitivity of the detection assay, however they limited the number of samples that could be withdrawn from the small-volume lower drug-receiving chamber. In order to increase the temporal resolution of the measured drug release kinetics, parallel experiments were performed so that in one set samples were taken at 5, 9 and 13 minutes and in another samples were withdrawn at 7, 11 and 15 minutes. Each of these experiments was repeated for each concentration of applied dobutamine 8 times.
Fig. 1. In vitro characterization of novel epicardial (EC) alginate drug release platforms to precisely control delivered dose rate.
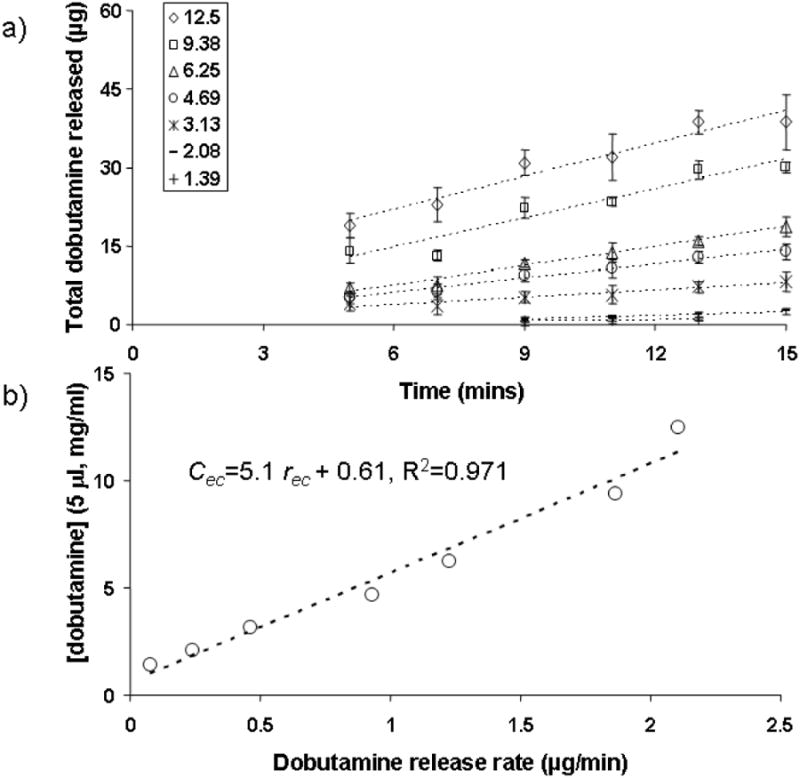
A) dobutamine (5 μl) is applied to the upper side of 6.5 mm diameter alginate discs in varying concentrations (1.39, 2.08, 3.13, 4.69, 6.25, 9.38 and 12.5 mg/ml) and the released drug into the lower receiving chamber is shown as a function of time (N=8 ±SD). Linear correlation of these data yielded a release rate for each applied concentration. B) The applied dobutamine concentration is shown as a function of release rate. Linear regression of these data allow the estimation of the appropriate concentration of drug in 5 μl aliquots (Cec) to be applied to the alginate discs in vivo to achieve a desired epicardial dobutamine release rate (rec).
The concentration of dobutamine in each in vitro release sample was determined by spectrophotometric methods.11 Briefly, metaperiodate (NaIO4, 6 μl, 2%, Sigma-Aldrich#S1878, St.Louis, MO) and ethanol (9 μl, 100%) were added to each sample and the absorbance at 490 nm was measured and compared to a standardized curve which was linear between 1 and 80 μg/ml. All of the in vitro data fell within this range. The release rates from 5 to 15 minutes were determined through linear regressions. These release rates were linearly correlated to the applied dobutamine concentration in 5 μl volumes Fig. 1b). These data allowed us to calculate a concentration of dobutamine to apply in 5 μl aliquots (Cec) to the upper surface of the alginate disc in vivo and thus dictate epicardial release rates (rec).
Rodent Preparation and Catheterization
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Massachusetts Institute of Technology, Cambridge MA and at St. Elizabeth's Medical Center, Boston, MA.
Male Sprague Dawley rats (400-450g) were anesthetized with pentobarbital (50 mg/kg, i.p.). Heat lamps and heating pads were adjusted to a rectal temperature of 37 °C. The neck, thorax, abdomen and inguinal areas were shaved and prepped with alcohol. An intraperitoneal catheter delivered continuous pentobarbital (30 mg/kg/hr). The right femoral artery and right internal jugular vein were accessed by a cut-down approach; the vessels were cleaned of fascia, isolated with 4-0 ligature and catheterized with polyethylene-50 tubing for arterial pressure monitoring and venous access, respectively. A midline incision over the neck allowed a tracheotomy and endotracheal intubation with a blunt 14 gauge catheter. The animals were ventilated by applying oxygen at 153 ml/min through a solenoid valve controlled by a computer (Labview Express 7.0, National Instruments, Austin, TX), a respiratory rate of 96 breaths per minute, an I:E ratio of 1:3 and an approximate tidal volume of 1.6 ml. A Millar pressure-volume catheter (6 mm, Millar catheter, #SPR-869, MPVS-300 system, Houston, TX) was introduced through a right carotid arteriotomy and into the left ventricle. The heart was exposed through bilateral vertical thoracotomies and any bleeding vessels were cauterized. Albumin (10%, Sigma-Aldrich#A7906) was infused through the jugular vein in 0.25 cc increments until the diastolic volume was adequate and the shape of the pressure volume (PV) loops showed 4 distinct phases of the cardiac cycle (Fig. 2).6,12-14 The volume of infused albumin was nearly always around 1 ml.
Fig. 2. Sample pressure-volume loops before and after EC and IV dobutamine (0.5 μg/kg/min) treatment.
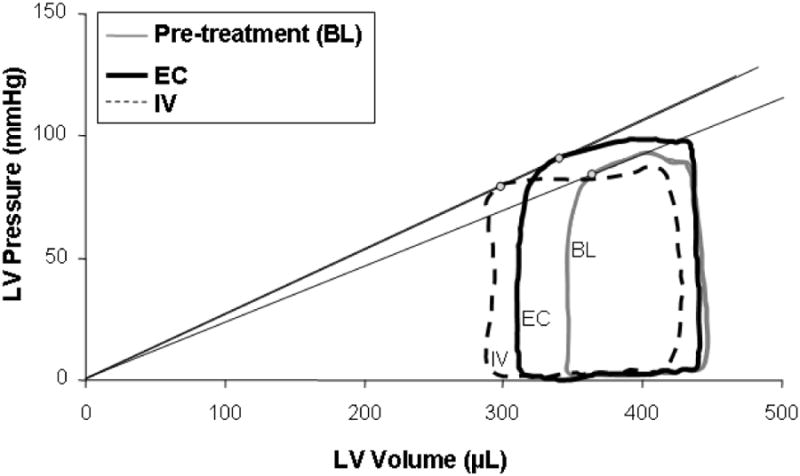
In this example, the LV pressure to volume ratio at end systole (Pes/Ves, circular markers), an index of contractility, increases similarly for EC (16%) and IV (14%) administration. The stroke volume (maximum less the minimum ventricular volume) is increased with both treatments from the pre-teatment baseline (BL), 27% for EC and 39% for IV. With EC application, left ventricular end diastolic volume (LVEDV) is mostly preserved, falling by less than 2%, while the pressure in the ventricle during systolic ejection is increased by 6%, indicating preserved preload and increased arterial pressures. With IV application, the LVEDV falls by 8% indicating decreased preload and the ventricular pressure during systolic ejection falls by 9% lower, indicating a loss of afterload and systolic arterial pressures. These pressure-volume loops suggest that the mechanism of action of inotropic agents depends on the method of application to the heart.
Data Acquisition
Continuous femoral arterial and central venous pressures (CVP) were transduced (Kent Scientific, TRN050, Torrington, CT) and amplified (Kent Scientific#TRN005, Torrington, CT). These analog signals, along with left ventricular pressure and volume signals from the Milar catheter, were acquired using a Power lab A/D converter (AD Instruments, Colorado Springs, CO) and fed into Chart 5.0 (AD Instruments, Colorado Springs, CO) software for instantaneous display, analysis and storage. Heart rate (HR) and maximum rate of change of left ventricular pressure per beat (max dP/dt) were derived from the pressure signal from the Millar catheter. Mean arterial pressure (MAP) was calculated from femoral arterial pressure tracings. Real time calculated hemodynamic parameters include relative stroke volume (SV) as the difference between maximum and minimum volume signals in each heart beat and systemic vascular resistance (SVR) calculated as the difference between the mean femoral and central venous pressures, normalized by the cardiac output (CO or HR × SV).
Drug Delivery
Dobutamine was given by intravenous (IV) infusion or locally through an epicardial (EC) alginate controlled release platform. For IV infusion, dobutamine was diluted based on the weight of the rat so that the infusion rate was always 0.2 ml/hr delivered by a syringe pump (Cole Parmer #74900-00) connected to the right internal jugular catheter. Saline was infused (0.2 ml/hr) during EC delivery and sham EC devices were placed on the heart during IV infusion. These sham EC devices ensured that any effects from device mass or potential free calcium release from the alginate on the contractility would be seen in both IV and EC treatment groups. Alginate EC drug releasing platforms were placed on the surface of the left ventricle with the concave side facing upwards and 5 μl of either drug solution or saline was applied to the top. The concentration of applied dobutamine was calculated from in vitro release data (Fig. 1b) to provide the desired drug release rate.
Continuous hemodynamic and Millar catheter data were recorded over 15 to 20 minutes, which pilot data suggested was long enough for the heart rate and max dP/dt signals to achieve steady state. The CVP was intermittently measured by halting the drug infusion with a 3-way stopcock for 3 seconds, which was not long enough to cause a disruption in dobutamine delivery at these low flow rates. Inotropic therapy often distorted the morphology of the pressure volume (PV) loops, due to relative motion of the catheter within the ventricle. Prior to making steady-state hemodynamic measurements, the Millar catheter was rotated slightly to restore the expected rectangular shape of the PV loops.6
In a group of 6 animals, EC devices were placed on the hearts, 5 μl of saline was added to the device and the hemodynamics recorded over 20 minutes. While there is some scatter in thse data, these negative controls showed that there was no measurable effect from the mass of the device, surface shear forces on the heart, nor any potential released free calcium (Table 1).
Table 1.
Impact of placebo alginate devices on hemodynamic and contractile indices on the anterior surface of the rat ventricle after 20 minutes (N = 6). All of these changes are far below the maximum changes seen with IV or EC dobutamine application, indicating minimal to no effects of the placebo alginate device.
| % change | SD | |
|---|---|---|
| Max dP/dt | -2.5 | 3.5 |
| HR | 6.2 | 9.4 |
| SVR | -4.5 | 9.5 |
| MAP | 6.0 | 7.7 |
| SV | -1.7 | 9.1 |
Dose Response (Sequential Multiple Dosing) Protocol
Epicardial and intravenous delivery of dobutamine was compared in two sets of experiments. In the first, multiple escalating doses were given to a single animal. After each dosing change, time was given for the animal to reach a new equilibrium as assessed by HR and blood pressure responses, usually within 15 minutes. Hemodynamic data was collected and the alginate device was replaced on the anterior surface of the heart prior to adjusting the delivered dose. Sequential dosing allowed us to characterize the dose-response in each animal but did not allow collection of pharmacokinetic data, as multiple doses would confound drug localization measurements and collection of blood and heart tissue samples required sacrificing the animal. Because the animals would not survive indefinitely, 3 to 6 measurements were made per animal. Data was collected at multiple doses between 0 and 5 μg/kg/min using 7 different animals for EC and 7 animals for IV application. 15-18
Pharmacokinetic (Single Dose) Protocol
In a second set of experiments, only one dose was given to each animal (0.5 or 5 μg/kg/min) by either EC or IV administration. This allowed us to acquire steady state tissue and blood samples for pharmacokinetic analysis. Following catheter rotation if necessary and hemodynamic assessment, a blood sample was taken from the femoral arterial catheter. The heart was quickly excised and rinsed with saline. Transmural cores (6 mm Miltex biopsy punches, VWR, Cat#21909-144) of tissue from the anterior wall of the left ventricle just under the alginate release device, the inferior wall, and right atrium containing the sinoatrial node were taken, rinsed of adsorbed drug in saline, blotted and snap frozen in cooled isopentane (-80 C). Blood samples were centrifuged at 2500 RPM for 15 minutes and the supernatant was frozen. Blood and tissue samples were sent to a commercial laboratory for drug concentration measurements through liquid chromatography – mass spectroscopy techniques (Agilux Laboratories, Worcester, MA).
Statistics
Statistically significant differences in hemodynamic and drug deposition data sets obtained with the Pharmacokinetic (Single Dose) Protocol were determined with two tailed non-paired Students t- tests, assuming unequal variance. Data was considered statistically distinct if the P value was less than 0.05. These statistical tests compared IV to EC application as well as two different doses of the same mode of delivery. Pharmacokinetic data that was below the detection limit of the LCMS method were not included in statistical analyses. Hemodynamic data as a function of resulting plasma dobutamine concentration was correlated to sigmoidal distributions (Prism 5.04, GraphPad, La Jolla, CA) which yielded minimal and maximum effects and the plasma concentration at 50% effect (EC50).19,20 Hemodynamic data taken with the Sequential Multiple Dosing Protocol were similarly fit to sigmoidal curves which yielded a maximum effect and dose at 50% effect (ED50).
Results
In vitro characterization
A novel experimental platform for controlling the epicardial release of inotropic drugs was developed for use in rats. Alginate was chosen for its ease in shaping in molds through calcium crosslinking. Preliminary data showed that a 2% starting alginate concentration yielded optimal mechanical properties. The solution was poured into a transwell support which was then immersed in a calcium. The meniscus from the original alginate solution in the transwell support formed a concave surface that was used to hold the drug solution and prevent it from spilling when applied to a beating rat heart.
Drug is applied to the upper surface of these concave devices in a single 5 μl application and the release is steady and linear over 15 minutes, and proportional to the concentration of the applied drug (Fig. 1). The applied dobutamine concentration (cec) correlates linearly with release rates (rec, Fig. 1b). By choosing the appropriate dilution for the applied drug, any desired release rate can be selected between 0.1 and 2.2 μg/min.
Pressure volume loop analysis
Example PV loops are shown for both EC and IV delivery before and after treatment (Fig. 2) and show that the same drug given by either systemic or local means have very different mechanisms of action. Both IV and PC treatments leads to increased stroke volume and contractility, as determined by the end-systolic pressure volume ratio (Pes/Ves).21,22 However, the left ventricular end diastolic volume (LVEDV) is lower in IV delivery, owing to systemic venodilation and subsequent decrease in venous return, ventricular filling and preload. LVEDV is relatively preserved in EC application indicating minimal effect on the venous system and therefore heart preload. Most importantly, the ventricular pressure during systolic ejection is decreased by IV infusion and increased in EC application, indicating loss of systemic vascular resistance, afterload and blood pressure in the former and preservation of arterial tone and blood pressure in the latter. While stroke volume may be enhanced more with IV infusion due to both higher contractility and reduced afterload, the associated drop in blood pressure may be deleterious in many clinical situations. Augmented contractility from EC application preserved preload and afterload and thus led to enhanced stroke volume with normotension. The sensitivity of these sample pressure volume loops to the method of application demonstrates differential pharmacology, mechanisms of action and side effect profiles and illustrate that local delivery has far more implications than simply targeted dosing.
Dose Response
EC delivery of dobutamine achieved the same net increase in myocardial contractility at significantly lower doses than was achieved IV. The maximum rise on max dP/dt, an index of myocardial contractility, is almost always greater for EC than IV delivery (Fig. 3A). Max dP/dt followed a classical sigmoidal fit, with an increase in peak effect of 49% and 62% for IV and EC application, respectively (Table 2). The ED50 for IV infusion and EC application was 1.7 and 1.1 μg/kg/min, respectively. Therefore, EC application of dobutamine is more efficacious and more potent than IV infusion at raising this index of contractility. At the highest dose (5 μg/kg/min) the rise in max dP/dt was statistically indistinguishable for IV and EC, but at modest doses (0.5 μg/kg/min) contractility with local EC application was statistically higher than IV infusion (Fig. 4A). At high dose, the average myocardial loading is sufficient to raise contractility, whether it is everywhere in the heart from IV infusion, or focused on one region of the left ventrcicle as might be observed with EC application. At low doses, systemic infusion is insufficient to load the tissue and increase contractility, whereas local EC application loads some part of the myocardial tissue enough to affect the measured global force of contraction.
Fig. 3. Dose response of dobutamine for IV and EC application in terms of A) max dP/dt, B) Heart rate (HR), C) systemic vascular resistance (SVR), D) Mean Arterial Preasure (MAP) and E) Stroke Volume (SV) over a wide range of applied doses.
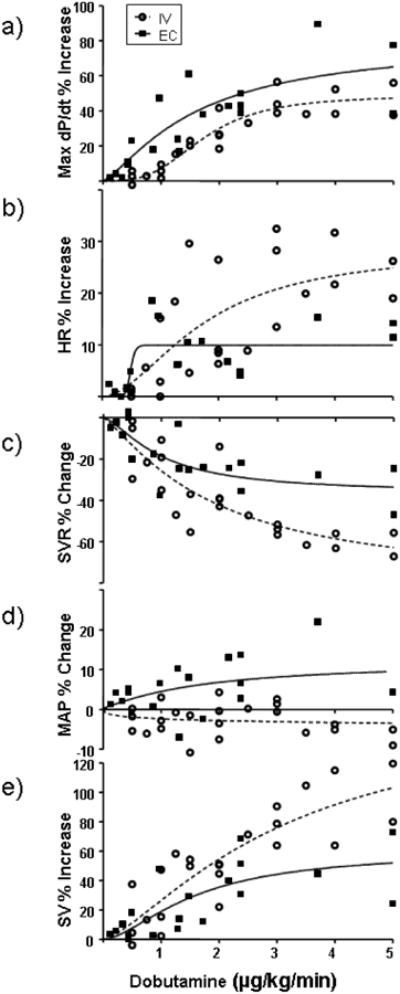
Data are fit to sigmoidal curves (Prism 5.04, Graph Pad). Peak increase in max dP/dt was 49% for IV and 62% for EC delivery. The ED50 for max dP/dt was 1.1 and 1.7 μg/kg/min for EC and IV, respectively. Peak increase in HR was 28% for IV and 10% for EC delivery. The ED50 for HR was 0.5 and 1.8 μg/kg/min, for EC and IV respectively. Peak decrease in SVR was 82% for IV and 39% for EC delivery. The ED50 for SVR was 1.0 and 1.9 μg/kg/min, for EC and IV respectively. Peak increase in MAP was 12% and the ED50 was 1.7 μg/kg/min for EC application. The MAP data for IV infusion did not fit a sigmoidal profile, however, most of the data show a reduction with dosing. Peak increase in SV was 158% for IV and 61% for EC delivery. The ED50 for SV was 1.6 and 3.2 μg/kg/min, for EC and IV respectively.
Table 2.
Peak effect and dose at 50% maximal effect (ED50) for measured hemodynamic and contractile indices following IV infusion or local EC application.
| IV | EC | |||||
|---|---|---|---|---|---|---|
| Max %percent; | ED50 (μg/kg/min) | R2 | Max %percent; | ED50 (μg/kg/min) | R2 | |
| Max dP/dt | 48.7 | 1.73 | 0.87 | 61.7 | 1.09 | 0.67 |
| HR | 28.5 | 1.75 | 0.50 | 9.96 | 0.49 | 0.47 |
| SVR | -82.1 | 1.89 | 0.70 | -39.0 | 1.05 | 0.56 |
| MAP | * | * | 12.3 | 1.73 | 0.15 | |
| SV | 158 | 3.17 | 0.74 | 61.0 | 1.64 | 0.52 |
Does not fit sigmoidal correlation.
Fig. 4. Change in A) max dP/dt, B) Heart rate (HR), C) systemic vascular resistance (SVR), D) Mean Arterial Preasure (MAP) and E) Stroke Volume (SV) at low.
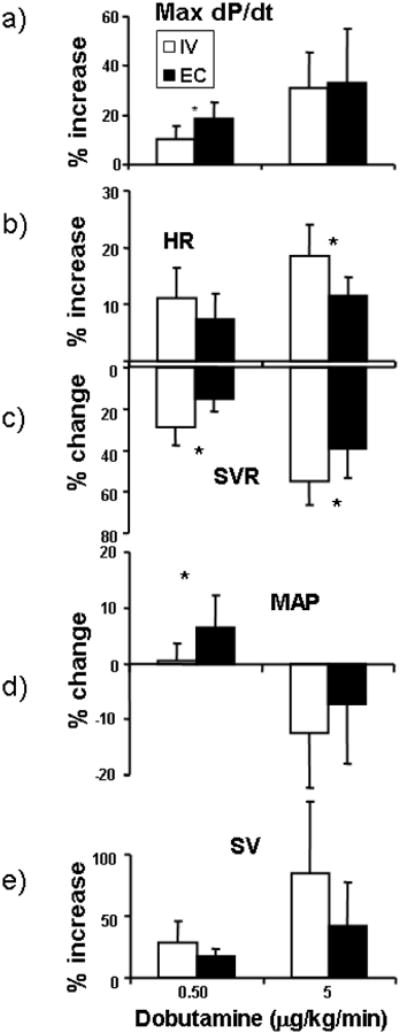
(0.5 μg/kg/min) and high (5 μg/kg/min) dose for EC and IV administration. At low dose the rise in contractility is significantly greater for EC over IV infusion, while at the higher dose both EC application and IV infusion increases contractility to a similar extent. HR increases more so for IV than EC application at the high dose and SVR falls to a greater extent with IV infusion than EC application at either dose. At low dose, MAP is higher for EC application than IV infusion, however, at higher doses MAP decreases similarly for both forms of delivery. Stroke volume increases in a dose dependent manner but the differences between EC and IV delivery were not statistically different at either dose (N=7, * P < 0.05).
Peripheral, non-myocardial effects were pronounced with IV and muted with EC dobutamine delivery (Table 2). HR increased exponentially with dose for IV application but far less after EC and without dose-responsiveness (Table 2, Fig. 3B). Single Dose data taken for pharmacokinetic analysis showed that HR is increased at the higher dose more so for IV infusion than EC application (Fig. 4b) Systemic vascular resistance (SVR) fell profoundly with IV dosing (Fig. 3C). SVR fell 48% as much with EC application and the effect plateaued at much lower doses. The ED50 for SVR was almost two-fold higher for IV than EC with values of 1.9 and 1.1 μg/kg/min, respectively (Table 2). Mean arterial pressure (MAP) decreased slightly at most IV doses, but rose with most EC doses (Fig. 3D). The stroke volume increased for both IV and EC application (Fig. 3E). Although the increase appeared greater in IV infusion, possibly from increased contractility and loss of systemic vascular tone, statistically these two methods of applying drugs to the heart were indistinguishable at low and high dobutamine dose (Fig. 4E). The combination of all of these data imply that there may be a range of optimal doses where the central contractile effects are maximized relative to systemic vasodilatory and chronotropic effects.
When data are plotted as the increase in myocardial contractility (max dP/dt) for dobutamine induced systemic effect, the difference in pharmacologic effects of IV and EC administration appears most pronounced (Fig. 5). While max dP/dt can be raised similarly with both routes of administration, IV infusion is associated with a much larger increase in HR and drop in SVR than seen with EC administration. Thus, the net effect of dobutamine on stroke volume was then greater with IV dosing but a good deal of this phenomenon was from peripheral vascular rather than myocardial effects – a set of responses that might not be tolerated in states of impaired vascular tone.
Fig 5. Scatter plots of contractility as a function of distant cardiovascular effects.
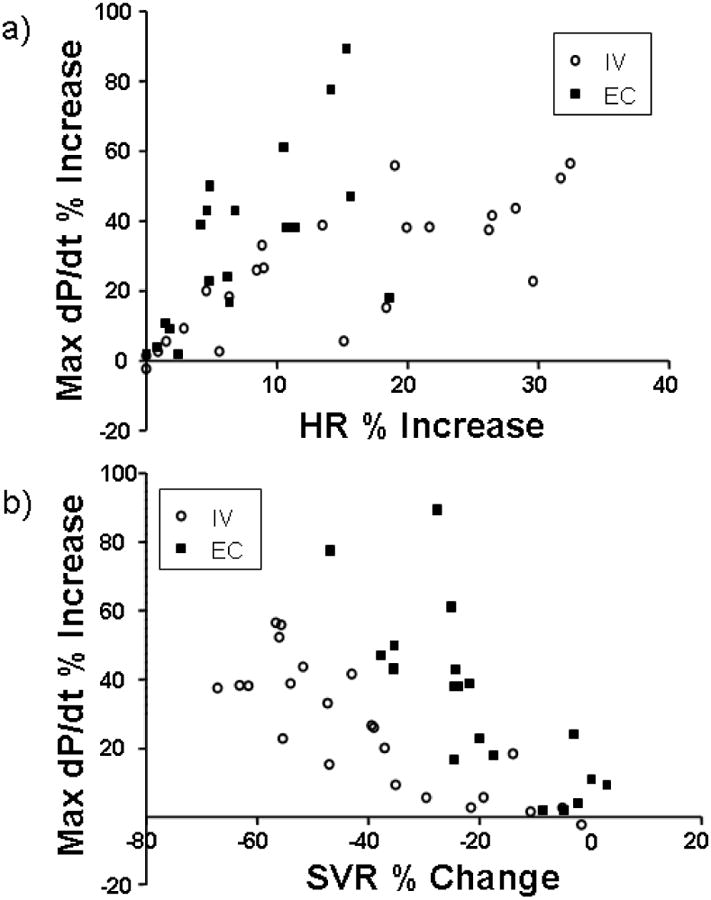
A) Heart rate (HR) response as a function of contractility response (Max dP/dt) for each data point in multidose per animal (dose response) protocol. For the same increase in contractility, IV infusion causes a greater increase in HR than EC application. B) Systemic vascular resistance (SVR) response as a function of contractility response (Max dP/dt) for each data point in multidose per animal protocol. For the same increase in contractility, IV infusion causes a greater loss of SVR than EC application.
Tissue Deposition and Blood Levels
Pharmacodynamic data taken in the single dose per animal protocol support the findings gleaned from the sequential dose protocol (Figs 3-5) and allow measurement of steady state plasma and heart tissue dobutamine concentrations (Fig. 6). The concentration of dobutamine in the anterior wall of the left ventricle was three orders of magnitude higher after local EC application than after IV infusion and was proportional to the applied local dose (Fig 6). Moreover, the myocardial tissue concentration in the anterior wall exceeded the circulating plasma concentration 523 fold with EC application, but was less than half the plasma concentration with given by IV infusion. The concentrations in the inferior wall of the left ventricle were below the detection limit for all applications except the high dose local application, which were orders of magnitude lower than for the corresponding concentrations in the anterior wall. The concentration of drug in the plasma following local EC application was 38% lower than with IV infusion at the highest dose. Although this difference was significant, the drug levels in the circulation were still detectable following local application. These data raise another potential fascinating implication of local delivery wherein intramuscular transport was more effective than intravascular, even in the face of heightened plasma concentrations. The right atrial concentration was statistically indistinguishable for all doses and modes of administration and for high dose IV therapy equivalent to the plasma concentration.
Fig 6. Pharmacokinetic data.
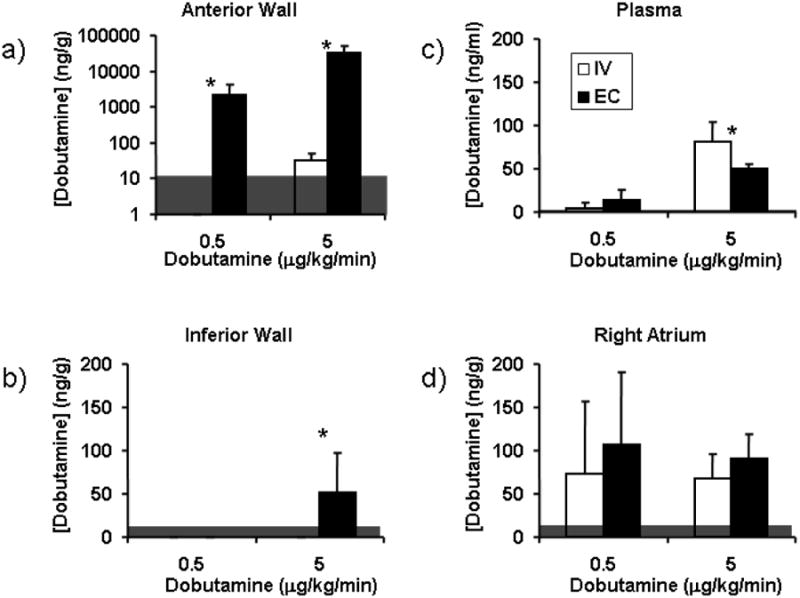
Dobutamine deposition in the A) anterior wall of the left ventricle, B) Plasma, C) Inferior wall of the left ventricle and D) right atrium. Tissue levels are normalized to mass of protein (g). The right atrium was sampled for dobutamine levels as a proxy for the concentration in the sinoatrial node that paces the heart. Some of the samples were below the detection limit (12.5 ng/g for tissue, 1 ng/ml for plasma, horizontal grey shading) of the liquid chromatography/mass spectroscopy technique (N=7, average ± SD, * P < 0.05).
It appears that significant amounts of dobutamine are detected within the plasma with high dose local EC delivery, albeit less than with IV infusion (Fig. 6b). At low doses, the plasma concentrations are statistically indistinguishable. Some of this systemic drug may play a role in both central inotropic and chronotropic, as well as peripheral vasodilatory effects. A strong correlation between the plasma concentration and a hemodynamic index would imply that systemic loading and not direct local application drove the effect. A strong correlation is seen between plasma concentration and max dP/dt, SVR, SV and MAP with IV infusion (Fig 7, Table 3). The correlation between plasma dobutamine concentration and HR was weaker. With EC application however, while SVR and MAP correlate strongly with plasma levels, max dP/dt, HR and SV do not. These data indicate that while plasma levels drive peripheral effects such as vascular tone regardless of the route of administration, effects that are dependent solely on myocardial drug levels, such as max dP/dt, are independent of circulating drug levels when given locally. Rather it is the transport of drug from controlled release device to the tissue that drives tissue deposition and local effect. SV on the other hand is governed by both peripheral and central effects and only correlates with blood levels when given systemically.
Fig. 7. Hemodynamic response as a function of plasma dobutamine concentration for A) max dP/dt, B) Heart rate (HR), C) systemic vascular resistance (SVR), D) Mean Arterial Preasure (MAP) and E) Stroke Volume (SV) for low.
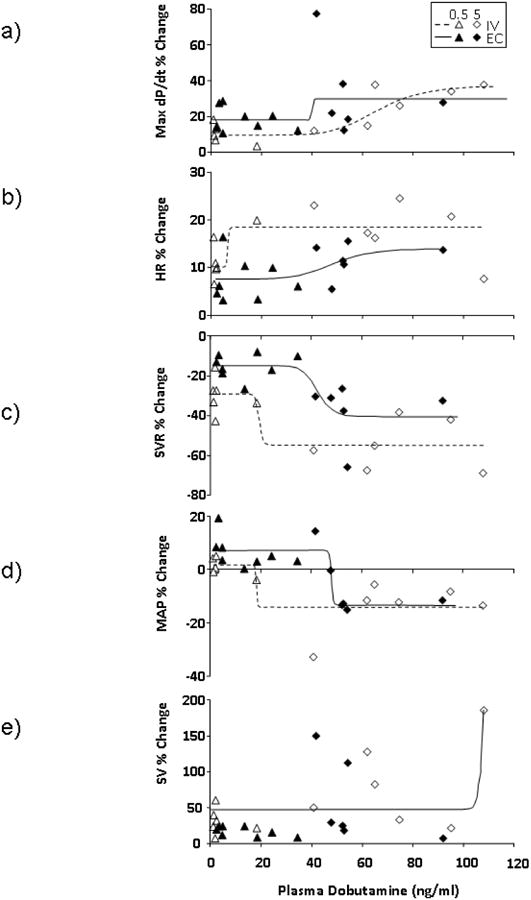
(0.5 μg/kg/min) and high (5 μg/kg/min) dose given either by EC or IV routes. Data are fit to sigmoidal curves (Prism 5.04, Graph Pad). Hemodynamic responses correlate to blood levels for IV infusion. Max dP/dt, HR and SV do not correlate with blood concentrations with local EC application, as they are dependent on drug levels within the myocardium. SVR and MAP, which can be considered peripheral effects correlate with plasma drug levels even with local application (N=7, note that some of the plasma samples were below detection limits and these data are omitted from this analysis.)
Table 3.
Minimal and maximal % change and plasma dobutamine concentration at half maximal transition (EC50) for measured hemodynamic and contractile indices following IV infusion or local EC application.
| IV | EC | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R2 | Min % | Max % | EC50 (ng/ml) | R2 | Min % | Max % | EC50 (ng/ml) | |
| Max dP/dt | 0.75 | 10.85 | 36.34 | 62.30 | 0.15 | 19.96 | 28.36 | 37.66 |
| HR | 0.42 | 10.65 | 18.43 | 6.66 | 0.23 | 7.60 | 14.09 | 48.73 |
| SVR | 0.61 | -29.35 | -54.95 | 19.36 | 0.59 | -15.05 | -40.64 | 42.42 |
| MAP | 0.58 | -14.01 | 1.84 | 18.41 | 0.79 | 7.28 | -13.25 | 47.98 |
| SV | 0.60 | 45.41 | ∼8.505e8 | 132.20 | * | |||
Does not fit sigmoidal correlation.
Discussion
Local therapy has become in vogue for many applications, specifically because local infusion may reduce systemic side effects and enhances selective delivery to a target organ. Our data add another dimension to the local therapy paradigm. Plasma concentrations should not differ substantially when the same drug is infused at the same dose from different sites unless there is significant local uptake of drug. Indeed, in our system, there was a statistically significant reduction in circulating dobutamine because there was markedly enhanced local tissue uptake and it is the local uptake that may explain the substantially different pharmacologic effect. IV infusion led to 63% more systemic drug in plasma than did EC application, as the latter extracted three-orders of magnitude more drug into the tissue. These findings expand our view of local drug delivery. The overall reduction in systemic levels was significant but modest and cannot likely account for a reduction in toxicity, but enhancing efficiency of local targeting and pharmacologic selectivity may offer a benefit of local delivery that transcends reduction of dose-related toxicity. The cardiovascular system is ideal for demonstrating this added effect of local delivery. The heart is highly vascular and its vasculature moves drug rapidly from the myocardium to the systemic circulation.2 The density of drug receptors, especially those that interact with the adrenergic nervous system, varies within the heart and is different from what is in blood vessels.23 The same adrenergic stimuli are controlled and mediated by different signaling pathways – stimulatory G proteins in the heart and both inhibitory and stimulating G proteins in the vasculature.3,4 Effects on the heart influence responses in the peripheral circulation and vice versa. Thus, a number of possible complimentary and synergistic effects can arise in the cardiovascular system.
Systemically administered drugs should present all organs with the same drug dose and same concentration gradient from perfusing vasculature to tissue. Local drug delivery can establish the same kind of systemic levels but very different concentrations gradients – extremely high at the point of drug release and in nearby tissues and far lower elsewhere.24,25 Dose may dictate net effect but concentration gradients drive drug transport and it is then possible for the local and systemic infusions of the same drug at the same dose to attain similar plasma levels but very different target organ levels. Indeed, our data shows that hemodynamic indices that depend on myocardial concentration such as max dP/dt and stroke volume do not correlate with plasma levels when given locally (Fig. 7). This is the departure from classic local delivery dogma as it presents a benefit to local delivery even when there is still significant plasma detection of drug.
The studies in the heart presented herein add even further to the complexity of local delivery and differential tissue uptake. The three-orders of magnitude greater amount of drug deposited in the anterior wall of the left ventricle with EC as opposed to IV infusion is interesting in confirming superior uptake; that there is little drug in the inferior wall is intriguing. These latter findings suggest that deposition of drug within myocardium is dominated by forces far beyond passive diffusion and may point to active modes of elimination consistent with observations related to contractile-dependent drug uptake.26,27 Local delivery is then far more powerful than a dose-limiting mode of administration and has the potential to add to the potency of clinical pharmacotherapy despite the complexity of resulting pharmacokinetics.
Alginate Epicardial Inotrope Releasing Platform
Systemic infusion is simple, requiring only intravascular access for delivery and its effect is rarely dependent on factors beyond dose, volume of distribution and clearance. Time-extended local drug delivery is predicated on having far greater control over administration but at far greater pharmacokinetic complexity. In addition to the factors in any dosing mode, there is now the need to define and control vehicle contact area without migration, integrity of adhesion without injury, rate of delivery and local tissue uptake, transport, distribution, metabolism and elimination. Controlled release devices that work by erosion or elution can meet these criteria but would require a different formulation for each drug, dose and clinical application. While not intended to be a useful clinical polymeric drug delivery system, the epicardial alginate discs used in this study provide a flexible, reliable method for studying mode of administration and can be used across a range of species of all sizes and for a spectrum of drugs at a series of doses. Preliminary optimization of the alginate platform entailed determining the density, area and thickness to allow for linear release over the time frames we were interested in, yet provided optimal mechanical properties of a flexible robust device that would not move about the surface of the heart. The alginate disks adhere to underlying tissue and remain in place through autoadhesion with no demonstrable adverse tissue effects to deliver drug to contact-tissue surfaces. The rate of release from the alginate devices is constant over the duration of the experiment, zero- ordered, easily and precisely controlled and allows delivery of a weight-based dosing regimen directly to the heart (Fig. 1). Linear drug delivery kinetics are obtained over a twenty-fold range of doses. While in vitro release can never fully predict in vivo release, the rapid motion of the heart may approximate the infinite sink conditions of the in vitro characterization. While these devices have little clinical utility as they erode within hours in the asbcence of calcium, they are ideal for short term delivery and can be adapted easily for other drugs and other species.
Local application is pharmacologically distinct from IV systemic infusion
Inotropic drugs, such as beta-adrenergic agonists like dobutamine, have receptors on cardiac myocytes and on arterial smooth muscle cells. Activation of these receptors in ventricular tissue increases inotropy (contractility), the force of myocardial contraction and on this basis raises stroke volume and cardiac output. Activation of beta receptors in cardiac impulse-generating nodes and conduction system stimulates chronotropy and heart rate. Beta adrenergic receptors in the peripheral vascular system relax smooth muscle and dilate arterial and venous blood vessels. Arteriodilation reduces systemic vascular resistance, the cardiac afterload. Venodilation increases venous capacitance and reduces venous return and cardiac preload. Moreover, the net physiologic response is a complex integration of interconnected reflexes. Changes in pressure and heart rate affect each other; stroke volume alterations can affect contractile demand and preload changes dynamically with cardiac work demand and diastolic filling. Thus, there are few pure effects in cardiac dynamics. What we did observe however was a difference in the dominant hemodynamic effects commensurate with enhanced local uptake following EC application. Local delivery increases local uptake and reduces direct loading of the plasma and hence peripheral vessels. As a result, the same dose of dobutamine elicited the same effect on contractility but with dramatically different systemic effects. SVR fell with increased contractility after EC delivery, but only at doses where contractility increased maximally (Fig. 5). It is possible that this arterial dilation is mediated by neurological reflexes in response to increased contractility from localized dobutamine in the heart. Systemic IV infusion, in contrast to EC application, reduced SVR far further and at lower doses where contractile effect was not yet maximized and likely represents a direct action on the vasculature in addition to neurological vasodilating reflexes to enhanced contractility.
Similarly heart rate was minimally elevated with EC application and rose statistically significantly with IV infusion (Figs. 3b & 4b). Thus, there is a two-fold effect on cardiac indices – a series of reflex effects from the primary pharmacologic recruitment of myocyte contraction and a direct pharmacologic effect on other tissues, in this case the sinoatrial pacemaker cells. Local therapy enhances the former while systemic therapy elicits the latter especially at higher doses. The benefits of local application extend then far beyond reduction of toxicity to controlled specificity and enhanced primary effect.
Drug Transport, Distribution and Elimination
The concentration of dobutamine varied for EC and IV delivery and in different parts of the heart and plasma (Fig. 6). The concentration of drug within the right atrium and anterior left ventricle after IV infusion were similar but virtually no drug was detectable in the inferior ventricular myocardium. Direct contact via EC delivery enhanced myocardial localization by three orders of magnitude. These data suggest a regional variation in drug deposition around the circumference of the heart and transport forces that transcend passive diffusion alone.
A significant amount of drug is noted in plasma following local EC application relative to IV infusion at high dose (5 μg/kg/min) (Fig. 6b). This raises the questions as to how drug is transported to the systemic circulation, and whether local EC application simply loads the circulation which then brings drug to the myocardium to produce pharmacodynamic responses. Drug can be absorbed by the circulation either through capillaries in the myocardium, capillaries in other thoracic structures, or across ventricular wall and into the left ventricular chamber. The high concentration of drug in the anterior wall and low concentration in the inferior wall suggests that drug moves directly to the tissue from the alginate discs and not through other thoracic structures. The anterior wall deposition suggests a high driving gradient into the myocardial capillaries of this highly perfused tissue. These capillaries may serve as drug sinks across the tissue and may limit the endocardial concentration.1,2 The low endocardial concentrations makes clearance to the left ventricular cavity less likely to be significant. Therefore, the most likely route of for drug from the point of release to the circulation is through the target myocardium. Thus, systemic drug levels may be an unavoidable consequence of local application to vascular organs such as the heart.
How significant then is plasma dobutamine concentration to driving myocardial pharmacodynamic response following EC application? SVR and MAP correlate strongly with plasma dobutamine levels while contractility (Max dP/dt), HR and SV correlate poorly (Fig. 7, Table 3). This implies that with EC delivery, circulating drug levels govern peripheral effects. Hemodynamic indices that reflect the state of the myocardial cells, such as max dP/dt, do not correlate with plasma drug levels implying that the dominant driver of intrinsic myocardial pharmacodynamic effect comes from the direct transfer of drug to target tissue and that circulating drug makes a minor contribution if at all.
The difference in deposition following local application between the anterior and inferior myocardial structures is a fascinating and yet unreported observation that begs further examination. Transmural and circumferential concentration gradients may exist and it is unclear the extent to which diffusive mechanisms and the coronary vasculature play roles in distribution from a point source on the surface. There are significant differences in beta adrenergic receptor density in different parts of the heart,23,28,29 distribution of degrading enzymes and local metabolism. Catechol-O-methyltransferase (COMT), the primary enzyme responsible for dobutamine degradation,30,31 is ten fold less active in the right atrium than in the left ventricle.32 Other studies have shown a regional variation in COMT activity in different chambers of the heart.33 In addition, 3-O-methyldobutamine, the metabolite from COMT activity does not appear to have any adrenergic properties.34 Red blood cells that course through the dense network of myocardial capillaries play a significant role in removing and degrading catecholamines from the tissue.35-37 The variation in degrading enzymes around the heart adds to a complex interplay between regional drug distribution, drug binding, clearance from capillaries and metabolism within the tissue. Local application will create drug concentration gradients within tissues which lead to transport, distribution and elimination that is complex and the deposition will be distinct from the deposition that results from IV infusion.
Future elucidation of the details of each of these processes may lead to optimal balancing of drug delivery, drug binding and degradation to achieve truly local effects with minimal peripheral consequences from systemic drug concentrations. More finessed, high resolution assessment of contraction, such as strain tensor MRI or speckle tracking echocardiography, may demonstrate that regions of the heart closer to the controlled release device may show localized contractile effect that is sufficient to carry and support the remainder of the left ventricle, as measured by a global index of contractility, such as max dP/dt. Alternatively, high resolution imaging may show that regional treatments with inotropic compounds may lead to mechanical inefficiencies that need to be overcome to achieve the desired global ventricular effect. Our data show that delivery of dobutamine to one region of the heart can augment global measurements of contractility to the same extent as IV infusion. Perhaps more spatially uniform EC drug delivery methods and better distribution would drive global ventricular indices of contractility such as max dP/dt even higher.
Clinical Implications
Patients in heart failure live in metastable, precarious states and are at risk for frequent acute events that decompensate cardiac function. Intravenous inotrope infusion can be of great benefit to patients in end-stage congestive heart failure, who need both augmentation of their heart contractile function and reduction of afterload to improve stroke volume and cardiac output. IV inotropes, however, are associated with tachycardia, reduced peripheral vascular tone and hypotension. These side effects may limit the dose of IV inotropic therapy. Furthermore, cardiac surgery is often vital for those with heart failure and yet cardiopulmonary bypass imposes especially high risks for those with reduced ventricular function. Cardiac surgical patients are most often already in a state of low vascular tone from a myriad of factors including circulating inflammatory mediators from the surgery, from blood exposure to synthetic surfaces in the CPB circuit and cellular damage in the CPB pump.38-40 The additional degradation of vascular tone imparted from IV inotropic compounds may cause unacceptable hypotension without a significant co-infusion of other agents, such as phenylephrine, norepinephrine, or vasopressin to raise blood pressure.41 These vasoconstricting agents may raise central blood pressure and organ perfusion, at the expense of vulnerable tissue beds, such as those fed by arteries with residual stenoses, or arteries prone to spasm including new coronary artery bypass grafts. The optimal use of inotropic compounds in surgical patients may require a delivery method wherein the patient experiences all of the positive inotropic effects without the negative effects of tachycardia and hypotension. Local epicardial delivery of inotropic drugs can achieve a distinct pharmacologic response from systemic IV infusion, with enhanced contractility with minimal peripheral vascular effects, changes in heart loading conditions, or deleterious peripheral side effects. EC inotropic treatments may support cardiac surgical patients' ventricular function throughout the perioperative period and may lead to easier and more successful separation from cardiopulmonary bypass without co-infusion of vasoconstricting agents. Current IV inotropic formulations do improve myocardial contractility but postoperatively require careful titration from highly skilled nurses in expensive intensive care environments. Even then, titration is problematic and haphazard. The lack of inherent peripheral side effects associated with EC inotropic therapy may allow safer care of these patients in more cost-effective settings. This technology could also be applied through minimally invasive access ports to the pericardial sac to provide long-term therapy for patients with chronic heart failure.42-46 EC treatments, while simple, straightforward and potentially minimally invasive, could radically alter therapeutic options, allow for safer surgery and postoperative management and help the prognosis and quality of life for many.
Conclusions
We have demonstrated a novel system for controlling the epicardial application of inotropic compounds. This demonstration of differential local uptake and effect after local delivery even in the face of detectable plasma drug levels from clearance from myocardial capillaries may add to our understanding of targeted and directed drug delivery. In the same vein the difference in effects on the heart and blood vessels highlights further the complexity of cardiovascular physiology and the clinical potential of local drug delivery in cardiovascular pathologic states.
Acknowledgments
This work was supported by the Desphpande Center for Technical Innovation at the Massachusetts Institute of Technology, a Scientist Development Grant from the American Heart Association and a Society of Cardiovascular Anesthesiologists Starter Grant.
References
- 1.Mak M, Fung L, Strasser JF, Saltzman WM. Distribution of drugs following controlled delivery to the brain interstitium. J Neurooncol. 1995;26(2):91–102. doi: 10.1007/BF01060215. [DOI] [PubMed] [Google Scholar]
- 2.Le KN, Hwang CW, Tzafriri AR, Lovich MA, Hayward A, Edelman ER. Vascular regeneration by local growth factor release is self-limited by microvascular clearance. Circulation. 2009;119(22):2928–2935. doi: 10.1161/CIRCULATIONAHA.108.823609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J Biol Chem. 2002;277(34):31249–31256. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 4.Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG. G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophys J. 2000;79(5):2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minneman KP, Hegstrand LR, Molinoff PB. Simultaneous determination of beta-1 and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol Pharmacol. 1979;16(1):34–46. [PubMed] [Google Scholar]
- 6.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, Kollai M, Szabo C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287(5):H2132–2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3(9):1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12(7):619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 9.Skjak-Braek G, Grasdalen H, Smidsrod O. Inhomogeneous Polysaccharide Ionic Gels. Carbohydrate Polymers. 1989;10:31–54. [Google Scholar]
- 10.LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res. 1999;47(1):46–53. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.el-Kommos ME, Mohamed FA, Khedr AS. Spectrophotometric determination of some catecholamine drugs using metaperiodate. J Assoc Off Anal Chem. 1990;73(4):516–520. [PubMed] [Google Scholar]
- 12.Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Liu J, Harvey-White J, Brassai A, Jarai Z, Cravatt BF, Kunos G. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2005;289(2):H533–541. doi: 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107(6):896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 14.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51(2):514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen JD, Frimodt-Moller N, Espersen F. Experimental Streptococcus pneumoniae infection in mice for studying correlation of in vitro and in vivo activities of penicillin against pneumococci with various susceptibilities to penicillin. Antimicrob Agents Chemother. 1995;39(6):1253–1258. doi: 10.1128/aac.39.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norenberg W, Hempel C, Urban N, Sobottka H, Illes P, Schaefer M. Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations. J Biol Chem. 2011;286(13):11067–11081. doi: 10.1074/jbc.M110.198879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez CA, Agudelo M, Zuluaga AF, Vesga O. In vitro and in vivo comparison of the anti-staphylococcal efficacy of generic products and the innovator of oxacillin. BMC Infect Dis. 2010;10:153. doi: 10.1186/1471-2334-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belz GG, Breithaupt-Grogler K, Butzer R, Fuchs W, Hausdorf C, Mang C. The pharmacological potency of various AT(1) antagonists assessed by Schild regression technique in man. J Renin Angiotensin Aldosterone Syst. 2000;1(4):336–341. doi: 10.3317/jraas.2000.063. [DOI] [PubMed] [Google Scholar]
- 19.Evans MA, Shanks CA, Brown KF, Triggs EJ. Pharmacokinetic and pharmacodynamic modelling with pancuronium. Eur J Clin Pharmacol. 1984;26(2):243–250. doi: 10.1007/BF00630293. [DOI] [PubMed] [Google Scholar]
- 20.Hockings N, Ajayi AA, Reid JL. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br J Clin Pharmacol. 1986;21(4):341–348. doi: 10.1111/j.1365-2125.1986.tb05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagawa K, Suga H, Shoukas AA, Bakalar KM. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol. 1977;40(5):748–753. doi: 10.1016/0002-9149(77)90192-8. [DOI] [PubMed] [Google Scholar]
- 22.Merillon JP, Neukirch F, Motte G, Aumont MC, Curien ND, Prasquier R, Gourgon R. The left ventricular end-systolic pressure-volume ratio. Studies during changes in load and inotropism in the human. Eur Heart J. 1981;2(1):41–48. doi: 10.1093/oxfordjournals.eurheartj.a061162. [DOI] [PubMed] [Google Scholar]
- 23.Burnam MH, Sethna DH, Rose DM, Stern CS, Shell WE. Differences in beta-adrenoceptor binding in anterior and inferior myocardial wall microsomes of normal canine left ventricle. Cardiovasc Res. 1981;15(4):239–244. doi: 10.1093/cvr/15.4.239. [DOI] [PubMed] [Google Scholar]
- 24.Lovich MA, Edelman ER. Tissue concentration of heparin, not administered dose, correlates with the biological response of injured arteries in vivo. Proc Natl Acad Sci U S A. 1999;96(20):11111–11116. doi: 10.1073/pnas.96.20.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creel CJ, Lovich MA, Edelman ER. Arterial paclitaxel distribution and deposition. Circ Res. 2000;86(8):879–884. doi: 10.1161/01.res.86.8.879. [DOI] [PubMed] [Google Scholar]
- 26.Wu PI, Edelman ER. Structural biomechanics modulate intramuscular distribution of locally delivered drugs. J Biomech. 2008;41(13):2884–2891. doi: 10.1016/j.jbiomech.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu PI, Minisini S, Edelman ER. Intramuscular drug transport under mechanical loading: resonance between tissue function and uptake. J Control Release. 2009;136(2):99–109. doi: 10.1016/j.jconrel.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan K, Bick RJ, Poindexter BJ, Nagueh SF, Shimoni S, Verani MS, Keng F, Reardon MJ, Letsou GV, Howell JF, Zoghbi WA. Altered adrenergic receptor density in myocardial hibernation in humans: A possible mechanism of depressed myocardial function. Circulation. 2000;102(21):2599–2606. doi: 10.1161/01.cir.102.21.2599. [DOI] [PubMed] [Google Scholar]
- 29.Shan K, Bick RJ, Poindexter BJ, Shimoni S, Letsou GV, Reardon MJ, Howell JF, Zoghbi WA, Nagueh SF. Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta-adrenergic receptor density in humans. J Am Coll Cardiol. 2000;36(3):891–896. doi: 10.1016/s0735-1097(00)00786-5. [DOI] [PubMed] [Google Scholar]
- 30.Yan M, Webster LT, Jr, Blumer JL. Kinetic interactions of dopamine and dobutamine with human catechol-O-methyltransferase and monoamine oxidase in vitro. J Pharmacol Exp Ther. 2002;301(1):315–321. doi: 10.1124/jpet.301.1.315. [DOI] [PubMed] [Google Scholar]
- 31.Yan M, Webster LT, Jr, Blumer JL. 3-O-methyldobutamine, a major metabolite of dobutamine in humans. Drug Metab Dispos. 2002;30(5):519–524. doi: 10.1124/dmd.30.5.519. [DOI] [PubMed] [Google Scholar]
- 32.Gennser G, Rosengren E, von Studnitz W. Distribution of noradrenaline and of monoamine oxidase and catechol-O-methyltransferase activity in human foetal heart. Experientia. 1973;29(1):20–22. doi: 10.1007/BF01913226. [DOI] [PubMed] [Google Scholar]
- 33.Krakoff LR, Buccino RA, Spann JF, Jr, De Champlain J. Cardiac catechol O-methyltransferase and monoamine oxidase activity in congestive heart failure. Am J Physiol. 1968;215(3):549–552. doi: 10.1152/ajplegacy.1968.215.3.549. [DOI] [PubMed] [Google Scholar]
- 34.Ruffolo R, Jr, Morgan EL. Interaction of the enantiomers of 3-0-methyldobutamine, a metabolite of dobutamine, with alpha- and beta-adrenoreceptors in the cardiovascular system of the pithed rat. J Auton Pharmacol. 1984;4(4):295–302. doi: 10.1111/j.1474-8673.1984.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 35.Alexander N, Velasquez M, Vlachakis ND. Red blood cells: in vivo site for transport and inactivation of biogenic amines in man and rats. Life Sci. 1981;29(5):477–482. doi: 10.1016/0024-3205(81)90214-9. [DOI] [PubMed] [Google Scholar]
- 36.Alexander N, Yoneda S, Vlachakis ND, Maronde RF. Role of conjugation and red blood cells for inactivation of circulating catecholamines. Am J Physiol. 1984;247(1 Pt 2):R203–207. doi: 10.1152/ajpregu.1984.247.1.R203. [DOI] [PubMed] [Google Scholar]
- 37.Yoneda S, Alexander N, Vlachakis ND, Maronde RF. Role of conjugation and red blood cells for inactivation of circulating normetanephrine. Am J Physiol. 1984;247(1 Pt 2):R208–211. doi: 10.1152/ajpregu.1984.247.1.R208. [DOI] [PubMed] [Google Scholar]
- 38.Carrel T, Englberger L, Mohacsi P, Neidhart P, Schmidli J. Low systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importance. J Card Surg. 2000;15(5):347–353. doi: 10.1111/j.1540-8191.2000.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 39.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26(5):1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Murphy GJ, Angelini GD. Side effects of cardiopulmonary bypass: what is the reality? J Card Surg. 2004;19(6):481–488. doi: 10.1111/j.0886-0440.2004.04101.x. [DOI] [PubMed] [Google Scholar]
- 41.St Andre AC, DelRossi A. Hemodynamic management of patients in the first 24 hours after cardiac surgery. Crit Care Med. 2005;33(9):2082–2093. doi: 10.1097/01.ccm.0000178355.96817.81. [DOI] [PubMed] [Google Scholar]
- 42.Laham RJ, Simons M, Hung D. Subxyphoid access of the normal pericardium: a novel drug delivery technique. Catheter Cardiovasc Interv. 1999;47(1):109–111. doi: 10.1002/(SICI)1522-726X(199905)47:1<109::AID-CCD24>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Verrier RL, Waxman S, Lovett EG, Moreno R. Transatrial access to the normal pericardial space: a novel approach for diagnostic sampling, pericardiocentesis, and therapeutic interventions. Circulation. 1998;98(21):2331–2333. doi: 10.1161/01.cir.98.21.2331. [DOI] [PubMed] [Google Scholar]
- 44.Waxman S, Pulerwitz TC, Rowe KA, Quist WC, Verrier RL. Preclinical safety testing of percutaneous transatrial access to the normal pericardial space for local cardiac drug delivery and diagnostic sampling. Catheter Cardiovasc Interv. 2000;49(4):472–477. doi: 10.1002/(sici)1522-726x(200004)49:4<472::aid-ccd28>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 45.Moreno R, Waxman S, Rowe K, Verrier RL. Intrapericardial beta-adrenergic blockade with esmolol exerts a potent antitachycardic effect without depressing contractility. J Cardiovasc Pharmacol. 2000;36(6):722–727. doi: 10.1097/00005344-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Hou D, Rogers PI, Toleikis PM, Hunter W, March KL. Intrapericardial paclitaxel delivery inhibits neointimal proliferation and promotes arterial enlargement after porcine coronary overstretch. Circulation. 2000;102(13):1575–1581. doi: 10.1161/01.cir.102.13.1575. [DOI] [PubMed] [Google Scholar]


