Abstract
Statement of the Problem
Generalized Anxiety Disorder (GAD) and disturbed sleep are prevalent, debilitating, and frequently comorbid problems for which successful treatment remains limited. Exercise can promote sleep but whether it does among GAD patients is unknown.
Methods
Thirty sedentary women (18–37y) with a primary DSM-IV diagnosis of GAD were randomized to six weeks of resistance (RET) or aerobic exercise training (AET), or waitlist (WL). RET and AET involved twice-weekly sessions of either lower-body weightlifting or leg cycling matched on multiple features of exercise. Outcomes included total sleep time (TST), lights out time, awakening out of bed time, time in bed (TIB), sleep onset latency (SOL), wakefulness after sleep onset, and sleep efficiency. Hedges’ d effect sizes and 95% confidence intervals were calculated for each exercise condition compared to WL. Regression examined baseline associations between anxiety and sleep and associated change.
Results
Twenty-two of 26 participants reported poor baseline sleep (Pittsburgh Sleep Quality Index >5). RET significantly decreased weekend TIB (d=−1.79; [−2.89, −0.70]) and SOL (d=−1.30; [−2.32, −0.28]), and significantly increased weekend sleep efficiency (d=1.30; [0.29,2.32]). AET significantly reduced weekend TIB (d=−1.13; [−2.16, −0.11]) and SOL (d=−1.08; [−2.09, −0.06]). Reduced GAD clinical severity rating was significantly associated with improved weekend sleep efficiency among RET (t6=−3.48, p≤0.013).
Conclusions
Short-term exercise training improves sleep outcomes among GAD patients, especially for RET and weekend sleep. Findings suggest improved sleep may be associated with reduced clinical severity among GAD patients.
Keywords: Resistance Exercise Training, Aerobic Exercise Training, Anxiety Disorders, Total Sleep Time, Sleep Onset Latency, Sleep Efficiency
Introduction
Generalized Anxiety Disorder (GAD) and disturbed sleep are prevalent, debilitating, and frequently comorbid public health problems for which successful treatment remains limited. Disturbed sleep may function as an antecedent, concomitant, or consequence of comorbid anxiety disorders (Jansson-Fröjmark & Lindblom, 2008; Johnson, Roth, & Breslau, 2006; Ohayon & Roth, 2003; Ramsawh, Stein, Belik, Jacobi, & Sareen, 2009). Though disturbed sleep is not required for diagnosis of GAD, the DSM-5 recognizes sleep disturbance as one of six independent, specific symptoms of GAD, and as many as 60% of GAD patients experience disturbed sleep (Papadimitriou, Kerkhofs, Kempenaers, & Mendlewicz, 1988). The available evidence from polysomnographic investigations suggests that GAD patients with mild-to-moderate intensity symptoms frequently experience prolonged sleep latency, lower total sleep time, and poor sleep efficiency (Monti & Monti, 2000; Papadimitriou & Linkowski, 2005). Much of the scant experimental evidence on sleep in GAD has focused on older adults or children and little is known about the sleep of young adults with GAD. Young adults are a population of interest because of their insufficient sleep and irregular sleep–wake patterns (e.g., staying out late on weekends) and because anxiety has been shown to account for most of the variability in their sleep quality (Lund, Reider, Whiting, & Prichard, 2010).
Pharmacotherapy and behavioral therapy have shown modest efficacy for improving symptom severity and disturbed sleep among GAD patients. For example, ten weeks of co-administered eszopiclone and escitalopram was associated with significant improvements in sleep, daytime functioning, anxiety, and mood among a large sample of patients meeting criteria for GAD and insomnia (Pollack et al., 2008). However, because GAD symptoms are heterogeneous, pharmacotherapy may attenuate one symptom yet exacerbate another. For instance, selective serotonin reuptake inhibitors, such as escitalopram, reduce GAD symptoms but have the capacity to exacerbate sleep disturbances (Schweitzer, 2000). Thus, similarly effective treatments with less risk of side effects (e.g., dizziness, loss of libido) are preferable. The first-line behavioral treatment, cognitive-behavioral therapy, presents significant barriers such as its expense and limited access to trained practitioners. Thus, there is continued interest in learning about inexpensive, alternative therapies for disturbed sleep among young adult patients with GAD.
A low cost and accessible potential therapy for disturbed sleep in GAD may be exercise. The sleep-promoting effects of exercise are well-established (King et al., 2008; Youngstedt, 2005). Evidence from survey and epidemiological research suggests that exercise may be a particularly useful behavior for sleep improvement (Youngstedt & Kline, 2006). Clinical trials of sleep apnea patients have shown significant reductions in apneas and hypopneas independent of reductions in body mass (Iftikhar, Kline, & Youngstedt, 2014; Kline et al., 2011). Reviews of experimental investigations in middle-aged and older adults with sleep problems have shown significant improvements in quality of life and sleep onset latency following exercise training (Yang, Ho, Chen, & Chien, 2012). In addition, reduced anxiety is a plausible mechanism through which exercise may influence sleep (Youngstedt, 2005). However, though exercise improves worry symptoms and the severity of GAD (Herring, Jacob, Suveg, Dishman, & O’Connor, 2012) along with associated signs and symptoms which also may be associated with disturbed sleep, (i.e., symptoms of anxiety, tension, depression, low vigor, fatigue, irritability, and pain) (Herring, Jacob, Suveg, & O’Connor, 2011), the effects of either aerobic or resistance exercise training on sleep among GAD patients are unknown.
Thus, the primary objective of the secondary analyses reported here was to quantify the magnitude of the effect of six weeks of either twice-weekly resistance or aerobic exercise training on self-reported sleep among sedentary young women with a principal diagnosis of GAD. As an exploratory objective we also examined baseline associations between anxiety and sleep and associated changes in response to exercise training.
Methods
Detailed methods of the overall randomized controlled trial have been reported elsewhere (Herring et al., 2012; Herring et al., 2011). The trial protocol was approved by the University of Georgia Institutional Review Board. Thirty sedentary women were recruited, agreed to participate, and provided written informed consent. Included women were: (1) 18–37 years of age; (2) engaged in no concurrent psychiatric or psychological therapy other than stable medication use; and (3) had a principal DSM-IV diagnosis of GAD. Women were excluded for: (1) too few worry symptoms, defined by both a Psychiatric Diagnostic Screening Questionnaire (Zimmerman & Mattia, 2001) GAD subscale score < 6 and a Penn State Worry Questionnaire (Brown, Antony, & Barlow, 1992) score < 45; (2) a highly physically active lifestyle, defined by a seven-day physical activity recall (7dPAR (Blair et al., 1985)) value > 260 kilocalories of energy expenditure per kilogram body weight per week; (3) pregnancy; and/or (4) medical contraindications to moderate-intensity exercise. Diagnostic interviews, including administration of the Anxiety Disorders Interview Schedule (ADIS-IV (Brown, Di Nardo, & Barlow, 1994)), were performed by clinicians blinded to treatment allocation. Potential participants assigned a clinician severity rating ≥ 4 were diagnosed with GAD, and were then enrolled in the intervention within 1–15 days following administration of the ADIS-IV. Patients were stratified based on psychotropic medication use (no medication vs. medication use) and randomized to conditions in blocks of three based on the intervention condition (resistance exercise training [RET], aerobic exercise training [AET], and waitlist control [WL]).
Conditions
Exercise conditions involved two sessions per week for six weeks. Sessions were conducted at approximately the same time of day, approximately 48 hours apart on Monday/Wednesday, Tuesday/Thursday, or Wednesday/Friday. Participants were instructed to otherwise maintain their normal lifestyle habits. As reported elsewhere, overall, weekday, and weekend extra-intervention physical activity was assessed with a 7dPAR (Blair et al., 1985; Herring et al., 2012).
Resistance exercise training
The RET condition involved seven sets of 10 repetitions each of leg press, leg curl and leg extension exercises beginning at 50% of predicted one-repetition maximum (1-RM) and progressing by five percent of predicted 1-RM each week (Herring et al., 2012). Each exercise was preceded by a warm-up set of 10 repetitions beginning at 35% of predicted 1-RM the first week and progressing by five percent of predicted 1-RM weekly. Heart rate and ratings of perceived exertion (RPE) were obtained following the completion of each exercise, and a session RPE was obtained following the completion of each workout.
Aerobic exercise training
AET was matched to the RET condition on four features of the exercise stimulus: (1) the time spent actively exercising, (2) the positive work completed during each exercise session, (3) a weekly five percent progression in load (intensity), and (4) a focus on lower-body exercise (Herring et al., 2012). AET involved two weekly sessions of 16 minutes of continuous, dynamic leg cycling exercise. Heart rate and RPE were obtained during each session at time points that approximated measurement during RET sessions.
Waitlist
Patients randomized to the WL condition delayed entry into any intervention for six weeks, but were tested on all outcomes. Following six weeks, each WL patient was offered a six-week exercise intervention, but no data were obtained during those sessions.
Sleep Data
The Pittsburgh Sleep Quality Index (PSQI) was completed at baseline to help characterize baseline sleep profile and sleep impairment among participants. At baseline and during the final week (week 6) of the intervention, sleep parameters were measured using the Pittsburgh Sleep Diary (PSD) (Monk et al., 1994). Each participant was instructed to complete the PSD daily for seven days. Outcomes of interest encompassed domains of sleep timing (lights out time, awakening out of bed time), duration (total sleep time [TST], time in bed [TIB]), and continuity (sleep onset latency [SOL], wakefulness after sleep onset [WASO], sleep efficiency [calculated as TST divided by TIB and expressed as a percentage]). Because of the potential health (Gaultney, 2014; Kim et al., 2012) and physical activity (Stone, Stevens, & Faulkner, 2013) consequences of differences between weekday and weekend sleep (Lauderdale, 2014), outcomes were examined separately for weeknights (i.e., Sun-Thur nights) and weekend nights (i.e., Fri-Sat nights). Diaries are considered the “gold standard” for subjective sleep assessment (Carney et al., 2012) and are modestly correlated with objective measures of sleep (e.g., actigraphy, PSG) (Kushida et al., 2001; Monk et al., 1994).
Anxiety and Worry Symptoms
The following measures were completed at baseline and post-intervention, as reported previously (Herring et al., 2012; Herring et al., 2011): clinical severity of GAD was quantified with the ADIS-IV Clinician Severity Rating (CSR) (Brown et al., 1994); anxiety symptoms, worry symptoms, and feelings of tension were assessed with the State-Trait Anxiety Inventory (Spielberger & Gorsuch, 1983), the Penn State Worry Questionnaire (Brown et al., 1992), and the Profile of Mood States – Brief Form (McNair, Droppleman, & Lorr, 1992), respectively.
Statistical Analyses
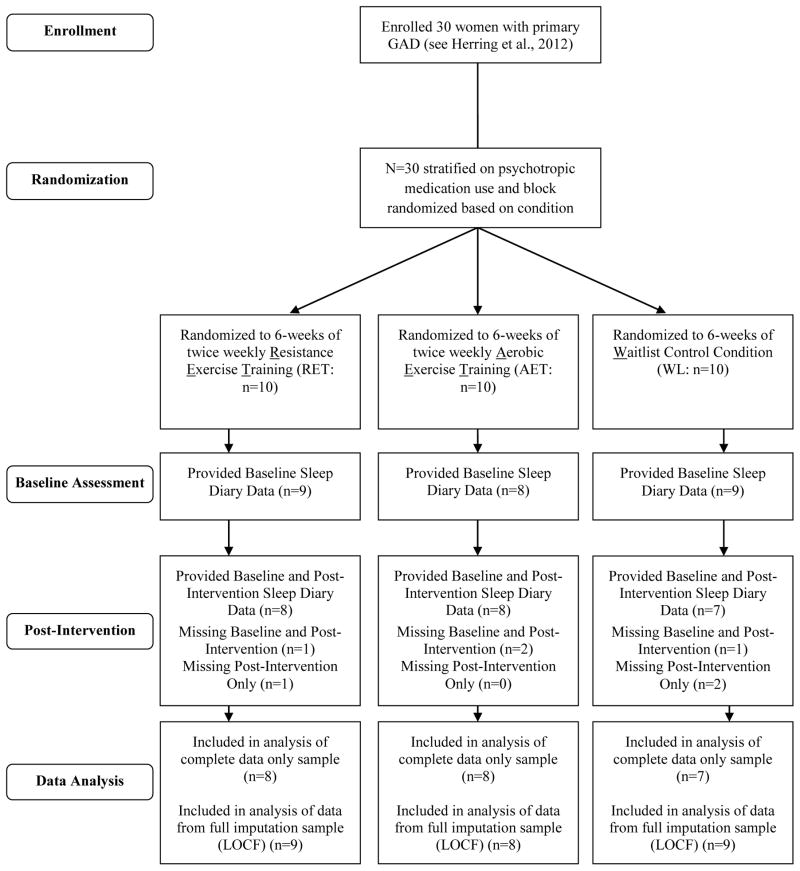
Figure 1 provides a CONSORT diagram of participant flow, data collection, and analysis. Of the 30 participants included in the overall randomized controlled trial (Herring et al., 2012), 23 provided complete baseline and post-intervention PSD data. An additional three participants (1 RET; 2 WL) provided baseline PSD data only and were included in analyses following Last Value Carried Forward imputation, yielding a total sample size of N=26 participants.
Figure 1.
CONSORT Diagram of Participant Flow Through Trial.
Potential baseline differences in sleep outcomes between conditions were examined using oneway ANOVA corrected for multiple testing (p≤0.005). Potential differences between participants who provided only baseline sleep data and participants who provided complete sleep were examined using oneway ANOVA corrected for multiple testing (p≤0.0045). Baseline associations of ADIS-IV GAD Clinical Severity Rating (CSR), worry symptoms (PSWQ), trait (STAI-Y2) and state (STAI-Y1) anxiety, and feelings of tension (POMS-B) with sleep outcomes were examined with regression models adjusted for condition and corrected for multiple testing (p≤0.005). Stratified models examined associations within each condition.
Hedges’ d effect sizes and associated 95% confidence intervals (95%CI) were calculated to assess the magnitude of treatment effects for RET and AET conditions compared with the WL condition. For each sleep parameter examined, the mean change from baseline for the WL condition was subtracted from the mean change from baseline for the RET or AET condition and the difference was divided by the baseline pooled standard deviation (Hedges, Olkin, Statistiker, Olkin, & Olkin, 1985). Each effect size was adjusted for small sample bias (Hedges et al., 1985). 95%CIs were examined to determine statistical significance.
Interaction coefficients for condition by GAD CSR, condition by worry symptom, and condition by anxiety symptom interactions were calculated and regression models corrected for multiple testing (p≤0.005) examined associations between changes in sleep and anxiety outcomes. Significant interactions were decomposed using follow-up models which examined associations between sleep and anxiety outcomes within each condition.
In addition, changes in overall, weekday, and weekend extra-intervention physical activity were examined using repeated measures ANOVA. Regression corrected for multiple testing (p≤0.01) examined associations of changes in weekday and weekend extra-intervention physical activity with change in sleep outcomes.
Results
Baseline Sleep
Table 1 provides baseline participant characteristics for the 26 participants included in these analyses, including baseline PSQI scores. At baseline, 22 of 26 (84.6%) participants reported PSQI scores >5 indicative of poor sleep quality.
Table 1.
Baseline Participant Characteristics
| Condition
|
||||
|---|---|---|---|---|
| RET (n=9) | AET (n=8) | WL (n=9) | Total (n=26) | |
| Age (y) | 26.3±7.1 | 20.4±2.5 | 24.7±6.5 | 23.9±6.2 |
| Weight (kg) | 60.6±10.2 | 65.5±9.2 | 66.2±9.0 | 64.1±9.5 |
| Height (cm) | 162.8±6.9 | 163.0±7.7 | 166.4±5.2 | 164.1±6.6 |
| BMI (kg/m2) | 22.8±3.1 | 24.7±3.6 | 66.2±9.0 | 23.8±3.3 |
| 7dPAR (kcal/kg/week) | 268.2±35.3 | 245.7±12.2 | 249.5±23.4 | 254.8±26.8 |
| 7dPAR Weekday | 197.2±31.5 | 180.1±11.9 | 181.3±19.2 | 186.4±23.2 |
| 7dPAR Weekend | 70.8±7.6 | 70.7±9.1 | 69.6±7.8 | 70.4±7.8 |
| Race (n) | ||||
| Caucasian | 5 | 6 | 6 | 17 |
| African-American | 0 | 1 | 1 | 2 |
| Hispanic | 2 | 1 | 0 | 3 |
| Asian | 2 | 0 | 2 | 4 |
| Psychoactive | 4 (44.4%) | 4 (50%) | 4 (44.4%) | 12 (46.2%) |
| Medication Use (n) | ||||
| SSRI | 2 | 2 | 2 | 6 |
| SNRI | 1 | 1 | 0 | 2 |
| NDRI | 1 | 1 | 1 | 3 |
| Muscle Relaxant | 1 | 1 | 0 | 2 |
| Antihistamine | 1 | 0 | 0 | 1 |
| Global PSQI Score (range: 0–21) | 8.8±4.2 | 10.8±3.3 | 8.8±4.7 | 8.8±4.2 |
| Number with poor sleep quality (PSQI>5; %) | 7 (77.8%) | 8 (100%) | 7 (77.8%) | 22 (84.6%) |
Abbreviations: 7dPAR: seven-day physical activity recall; SSRI: selective serotonin reuptake inhibitor; SNRI: Serotonin-Norepinephrine Reuptake Inhibitor; NDRI: Norepinephrine-Dopamine Reuptake Inhibitor; PSQI: Pittsburgh Sleep Quality Index.
Importantly, though participants were not formally evaluated for insomnia diagnosis, 13 participants (50%; 4 RET, 5 AET, 4 WL) reported baseline weekday SOL and sleep efficiency data which met quantitative criteria for insomnia (i.e., SOL≥ 20 min, sleep efficiency < 85%) (Lineberger, Carney, Edinger, & Means, 2006; Perlis et al., 2014). Nine participants (35%; 4 RET, 2 AET, 3 WL) reported weekend SOL and sleep efficiency data which met quantitative criteria for insomnia. Five participants (1 RET, 2 AET, 2 WL) reported both weekday and weekend SOL and sleep efficiency data which met quantitative criteria for insomnia.
PSQI score did not significantly differ between conditions (F(2,23)=0.55, p≥0.59). Weekday TIB differed between conditions (F(2,23)=5.29, p≤0.013); Bonferroni-corrected pairwise comparisons showed that TIB was higher among AET compared to WL (mean difference: 49.05, p≤0.02) and RET (mean difference: 44.34, p≤0.039). No statistically significant differences were found between conditions for any other sleep outcome. Baseline differences between conditions did not differ between the LVCF and completer-only samples.
Baseline weekday WASO was higher among participants who provided only baseline sleep data (25.92±15.48 min) compared to participants who provided complete sleep data (9.72±11.24 min; F1,24=5.13, p≤0.033). However, PSQI score, weekday and weekend SOL, weekend WASO, weekday and weekend TST, weekday and weekend TIB, and weekday and weekend sleep efficiency were not significantly different between participants who provided complete sleep data and participants who provided only baseline sleep data (all p>0.22).
Baseline Associations Between Anxiety and Sleep Outcomes
Eleven of the 12 participants in this sample who remitted (i.e., no longer met diagnostic criteria for GAD following exercise intervention; see (Herring et al., 2012)) reported poor sleep quality (PSQI>5) at baseline; 11 of 14 non-remitters reported poor sleep quality at baseline. Among RET participants, 7 of 9 (77.8%) reported poor sleep quality at baseline; 5 of 5 remitters and 2 of 4 non-remitters reported poor sleep quality. Among AET participants, 8 of 8 (100%) reported poor sleep quality at baseline; 4 of 4 remitters and 4 of 4 non-remitters reported poor sleep quality. For WL, 7 of 9 (77.8%) participants reported poor sleep quality at baseline; 5 of 6 non-remitters and 2 of 3 remitters reported poor sleep quality.
ADIS-IV Clinician Severity Rating (CSR) for GAD (RET: 4.89±0.60; AET: 4.88±0.83; WL: 5.67±0.87) was not significantly associated with PSQI score (t20=−0.35, p>0.73), independent of condition. Among the full sample of participants, PSQI score was not significantly associated with any anxiety-related outcome at baseline (all p>0.37).
Following correction for multiple testing (p≤0.005), no statistically significant associations were found between anxiety-related outcomes and sleep outcomes. Nominal associations are presented below. GAD CSR was positively associated with weekend TST (t20=2.25, p≤0.036, β=0.47) and TIB (t20=2.33, p≤0.03, β=0.49). A positive association was found between feelings of tension and weekend TIB (t20=−2.09, p≤0.049, β=0.42). Weekday and weekend SOL, WASO and sleep efficiency, and weekday TST and TIB were not significantly associated with any anxiety-related outcome in the full sample. Baseline associations did not differ between the LVCF and completer-only samples.
Intervention Fidelity
As previously reported (Herring et al., 2012), participants in all three conditions attended 100% of scheduled sessions; RET complied with 99.1% of the RET protocol (1 incomplete session), and AET complied with 100% of the AET protocol. RET was characterized by significantly higher RPE during exercise and session RPE compared to AET (all p<0.001), but heart rate did not significantly differ between conditions (Herring et al., 2012).
Change in Sleep Outcomes
Table 2 provides descriptive statistics by condition for each sleep outcome during weeknights and weekends at baseline and post-intervention along with Hedges’ d effect sizes (95%CIs) for RET and AET at post-intervention.
Table 2.
Effects of RET and AET on Sleep Parameters
| Baseline | Week 6 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Weeknight | Weekend | Weeknight | Weekend | |||
|
| ||||||
| Outcome | Mean (SD) | Mean (SD) | Mean (SD) | Hedges’ d (95%CI) | Mean (SD) | Hedges’ d (95%CI) |
| Total Sleep Time (TST; min) | ||||||
| RET (n=9) | 462 (27) | 466 (50) | 448 (17) | −0.78 (−1.74, 0.18) | 466 (62) | −0.90 (−1.87, 0.07) |
| AET (n=8) | 418 (44) | 475 (80) | 446 (53) | 0.18 (−0.77, 1.13) | 475 (81) | −0.72 (−1.70, 0.26) |
| WL (n=9) | 458 (50) | 492 (63) | 477 (121) | 546 (168) | ||
| Lights Out Time (Military Time; SD [min]) | ||||||
| RET | 23:42 (86) | 00:17 (87) | 23:57 (62) | 0.38 (−0.55, 1.31) | 00:40 (65) | 0.63 (−0.32, 1.57) |
| AET | 00:39 (67) | 01:46 (72) | 00:35 (76) | 0.22 (−0.73, 1.18) | 01:37 (106) | 0.31 (−0.65, 1.27) |
| WL | 23:54 (121) | 00:08 (97) | 23:27 (73) | 23:31 (161) | ||
| Awakening Out of Bed Time (Military Time; SD [min]) | ||||||
| RET | 08:15 (99) | 08:59 (87) | 07:59 (80) | 0.05 (−0.88, 0.97) | 08:47 (105) | −0.92 (−1.89, 0.05) |
| AET | 08:26 (50) | 10:23 (95) | 08:48 (65) | 0.38 (−0.58, 1.35) | 10:07 (107) | −0.85 (−1.84, 0.15) |
| WL | 08:34 (139) | 09:02 (113) | 08:13 (115) | 10:27 (180) | ||
| Time in Bed (TIB; min) | ||||||
| RET | 528 (29) | 528 (41) | 505 (37) | −0.86 (−1.82, 0.11) | 493 (59) | −1.79 (−2.89, −0.70)a |
| AET | 484 (38) | 535 (83) | 502 (62) | 0.31 (−0.65, 1.27) | 517 (83) | −1.13 (−2.16, −0.11)a |
| WL | 533 (35) | 554 (70) | 539 (117) | 627 (168) | ||
| Sleep Onset Latency (SOL; min) | ||||||
| RET | 28 (26) | 15 (11) | 20 (27) | −0.13 (−1.06, 0.79) | 12 (9) | −1.30 (−2.32, −0.28)a |
| AET | 26 (18) | 12 (12) | 19 (15) | −0.11 (−1.07, 0.84) | 11 (9) | −1.08 (−2.09, −0.06)a |
| WL | 25 (16) | 16 (11) | 20 (21) | 28 (39) | ||
| Wakefulness After Sleep Onset (WASO; min) | ||||||
| RET | 9 (8) | 14 (24) | 7 (8) | 0.08 (−0.85, 1.00) | 6 (10) | −0.27 (−1.20, 0.66) |
| AET | 12 (14) | 15 (21) | 21 (31) | 0.75 (−0.23, 1.74) | 7 (9) | −0.28 (−1.23, 0.68) |
| WL | 14 (16) | 18 (32) | 11 (14) | 18 (36) | ||
| Sleep Efficiency (%) | ||||||
| RET | 86.9 (6.7) | 87.1 (5.3) | 88.5 (7.5) | −0.04 (−0.96, 0.89) | 93.2 (4.8) | 1.30 (0.29, 2.32)a |
| AET | 86.6 (6.4) | 88.9 (7.6) | 89.0 (6.2) | 0.06 (−0.89, 1.02) | 91.7 (3.9) | 0.68 (−0.30, 1.66) |
| WL | 86.0 (8.1) | 89.1 (7.0) | 87.9 (6.1) | 86.7 (10.6) | ||
Abbreviations: SD, standard deviation; 95%CI, 95% confidence interval; RET, resistance exercise training; AET, aerobic exercise training; WL, waitlist
p < 0.05
Weekends
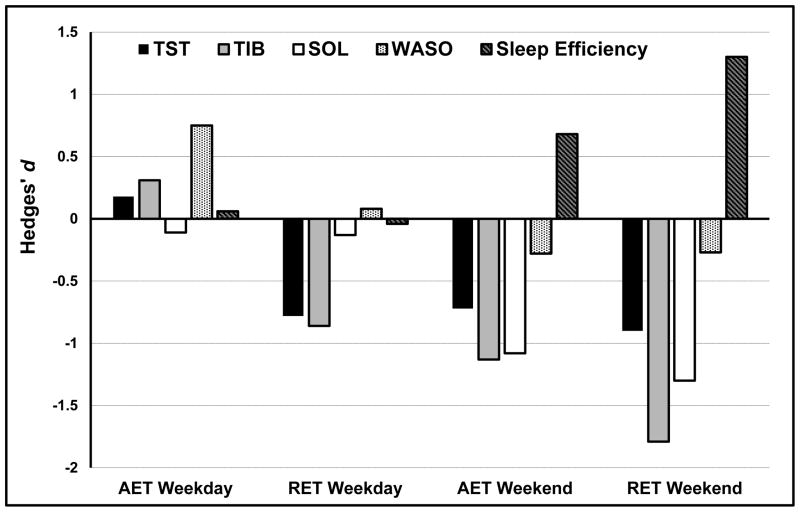
Figure 2 illustrates the magnitude of effects for RET and AET across weeknight and weekend sleep outcomes. Compared to WL, six weeks of RET significantly decreased weekend TIB (d = −1.79; 95%CI: [−2.89, −0.70]) and SOL (d = −1.30 [−2.32, −0.28]), and significantly increased weekend sleep efficiency (d = 1.30 [0.29, 2.32]). Compared to WL, six weeks of AET significantly reduced weekend TIB (d = −1.13 [−2.16, −0.11]) and SOL (d = −1.08 [−2.09, −0.06]). RET resulted in large, non-significant reductions in weekend TST (d = −0.90 [−1.87, 0.07]) and awakening out of bed time (d = −0.92 [−1.89, 0.05]). AET resulted in moderate-to-large, non-significant reductions in weekend TST (d = −0.72 [−1.70, 0.26]) and awakening out of bed time (d = −0.85 [−1.84, 0.15]) and a moderate-to-large increase in weekend sleep efficiency (d = 0.68 [−0.30, 1.66]). Of the 9 participants who met quantitative criteria for insomnia based upon weekend sleep at baseline, 4 RET and 2 AET no longer met these criteria at post-intervention.
Figure 2.
Effects of AET and RET on Weekday and Weekend Sleep.
Weeknights
No significant changes were observed for weeknight sleep outcomes. RET resulted in large, non-significant reductions in weeknight TST (d = −0.78 [−1.74, 0.18]) and TIB (d = −0.86 [−1.82, 0.11]). Of the 13 participants who met quantitative criteria for insomnia based upon weeknight sleep at baseline, 2 RET, 2 AET, and 1 WL participant no longer met these criteria at post-intervention. Of the five participants who met insomnia criteria based upon both weeknight and weekend sleep at baseline, 1 RET and 2 AET no longer met these criteria at post-intervention.
Results following LVCF imputation did not significantly differ from results for the completer-only sample; Hedges’ d for weekday sleep outcomes differed on average −0.04 and 0.05 between LVCF and completer-only for RET and AET, respectively, while Hedges’ d for weekend sleep outcomes differed on average −0.13 and −0.07 for RET and AET, respectively.
Associations of Change in Anxiety Outcomes & Change in Sleep Outcomes
Following correction for multiple testing (p≤0.005), a statistically significant condition by change in GAD CSR interaction was found for change in weekend sleep efficiency (t19=3.22, p≤0.004, β=1.66); follow-up models revealed that reductions in GAD CSR were significantly associated with improvements in weekend sleep efficiency among RET participants (t6=−3.48, p≤0.013, β= −0.82). No other statistically significant associations were found between change in sleep outcomes and change in anxiety outcomes; associations did not significantly differ between conditions. A nominally significant condition by change in worry symptom interaction was found for changes in weekday TST (t22=−2.97, p≤0.007, β= −1.19) and TIB (t22=−2.94, p≤0.008, β =−1.16); follow-up models revealed significant inverse associations between changes in worry symptoms and changes in weekday TST (t7=−2.38, p≤0.049, β= −0.67) and TIB (t7=−2.66, p≤0.033, β= −0.71) among WL participants. These findings did not differ between the LVCF and completer-only samples.
Overall, Weekday, and Weekend Extra-Intervention Physical Activity
Extra-intervention physical activity did not significantly change across six weeks; changes did not significantly differ between conditions; and, changes were not significantly associated with change in any sleep outcome (all p>0.25). Weekday physical activity did not significantly change across six weeks (p>0.56; RET: −4.4%±8.1%; AET: +5.4%±12.1%; WL: +3.8%±10.5%), and changes did not significantly differ between conditions (p>0.11). Weekend physical activity did not significantly change across six weeks (p>0.14; RET: −0.9%±7.7%; AET: +10.9%±18.6%; WL: +7.5%±22.0%), and changes did not significantly differ between conditions (p≥0.34).
Following correction for multiple testing (p≤0.01), change in weekday physical activity was not significantly associated with change in any weekday sleep outcome for any condition (all p≥0.046), and change in weekend physical activity was not significantly associated with change in any weekend sleep outcome for any condition (all p≥0.068). Stratified models showed a nominal inverse association between change in weekday SOL and weekday physical activity among WL participants (t7=−2.42, p≤0.046). These findings did not differ between the LVCF and completer-only samples.
Discussion
These preliminary findings suggest that short-term exercise training can improve sleep disturbance among patients with GAD. The most prominent exercise-related improvements found among young women with GAD included improved sleep initiation and sleep continuity, as well as reductions in time spent in bed and hypersomnia. Because there appear to be no prior studies of exercise training effects on sleep among adults with GAD to which the present findings can be compared, these results provide initial evidence that exercise training may be an efficacious nonpharmacologic therapy for this population. A larger trial that measured sleep symptoms with the Pittsburgh Sleep Quality Index that involved 12 weeks of aerobic exercise performed by perimenopausal and postmenopausal women with elevated GAD symptoms found small improvements in sleep quality and insomnia symptoms compared to controls (Sternfeld et al., 2014).
The present findings, particularly exercise-induced reductions in TST and TIB, highlight the potential importance of normalization of sleep pattern away from the hypersomnia frequently found among individuals with an anxiety and/or depressive disorder (Breslau, Roth, Rosenthal, & Andreski, 1996). Although speculative, reduced TST in response to exercise could have been a consequence of more continuous (and more restful) sleep. This finding reiterates the importance of consolidated sleep in contrast to an exclusive focus on sleep duration.
Weekend TIB decreased after both resistance (35min) and aerobic exercise training (18min) to 8.2 and 8.6 hours, respectively. A U-shaped relationship is commonly observed between TIB or self-reported TST and health, with the lowest risk typically between 7–8 hours of TIB or TST (Kurina et al., 2013). Thus, the reductions in TIB observed here did not approach short TIB durations (<6 hours) associated with obesity (Hart, LaRose, Fava, James, & Wing, 2013) or poor health behaviors such as smoking (Stea, Knutsen, & Torstveit, 2014). The waitlist group did show an increase in TIB during the trial to a long duration (≥=10.5 hours) that is associated with poorer health status (Sekine, Tatsuse, Cable, Chandola, & Marmot, 2014).
Approximately one-half of the sample met quantitative criteria for insomnia based on weeknight sleep at baseline. Weekend mean self-reported SOL showed an absence of long SOL, one of the manifestations of insomnia, for all the groups at baseline. The exercise groups improved in SOL by small amounts while the waitlist controls reported longer SOL at the end of the trial. These findings of reduced SOL are consistent with prior exercise training trials conducted with mid-aged and older adults (Yang et al., 2012).
Baseline sleep efficiency (86–89%) was lower than typical among healthy young adults (~95%) and significantly improved after aerobic and resistance exercise training (89%–93%). Greater sleep continuity is commonly associated with exercise in epidemiologic research (Kline et al., 2013), though experimental studies have been less consistent in finding improved sleep efficiency following exercise training (Kline et al., 2011; Yang et al., 2012).
In agreement with prior research, weekend sleep was longer and initiated with less difficulty (i.e., lower SOL) compared to weekdays (Breslau, Roth, Rosenthal, & Andreski, 1997; Buboltz et al., 2009). Moreover, the effects of both resistance and aerobic exercise training on sleep were largely restricted to weekend sleep, and these effects were not significantly associated with changes in weekday or weekend extra-intervention physical activity. One plausible explanation for these findings is that weekday sleep quality may be constrained in this sample of young adults, all of whom were full-time students whose reported sleep/wake schedules did not significantly differ (see Table 2 and results for lack of baseline differences between conditions), by external factors related to school/work which may contribute to higher stress (Tsai & Li, 2004). Differences between exercise-induced changes in weekday and weekend sleep merits further inquiry.
Anxiety reduction is commonly postulated as a mechanism by which exercise improves sleep (Youngstedt, 2005). To our knowledge, this is the first study to demonstrate associations between reduced anxiety and improved sleep in a sample of individuals with GAD. Though others have observed reduced anxiety and improved sleep following acute exercise (Passos et al., 2010) or exercise training (Passos et al., 2011), these parameters were not significantly correlated in these studies. Although our results do not address whether reduced anxiety led to better sleep or vice versa in this sample, the relationship between anxiety and sleep is thought to be bidirectional (Alvaro, Roberts, & Harris, 2013). Exercise may improve sleep through mechanisms other than anxiety reduction which may have occurred here but were unable to be tested.
Limitations
The small sample size of 26 for this pilot trial is a limitation, though statistically significant moderate-to-large improvements in sleep outcomes were found. In addition, though matching on multiple features of the exercise stimulus to facilitate experimentally rigorous comparisons of the effects of resistance and aerobic exercise is a novel strength beyond the previous literature, the resulting exercise dose, particularly for aerobic exercise training, is somewhat low. Future studies may benefit from examining a more intense exercise stimulus to evaluate possible dose-response relations with sleep (Kline et al., 2012). The present findings are based on a self-report diary measure of sleep; objective measurement of sleep, including actigraphy or polysomnography, would provide a more rigorous examination of the effects of aerobic and resistance exercise training. Though measuring sleep during the final week of the intervention may introduce a limitation via the potential influence of acute effects of exercise, it is unlikely that the findings for weekday sleep responses to chronic exercise training, which are based on the mean of five days, are explicitly attributable to sleep reports on the two nights following exercise during the final week of the trial. Nonetheless, the present findings suggest that as few as six weeks of resistance or aerobic exercise training can elicit significant, moderate-to-large improvements in sleep among young women with GAD.
Conclusions
The present findings suggest that short-term exercise training improves self-reported sleep outcomes among GAD patients. Improvements were stronger in response to resistance exercise and during the weekend when sleep may not be as constrained by external demands. Findings suggest improved sleep may be associated with reduced clinical severity among GAD patients.
Highlights.
Comorbid GAD and disturbed sleep are prevalent and often unsuccessfully treated
Thirty women with GAD were randomized to resistance or aerobic exercise or wait-list
Twenty-six participants provided Pittsburgh Sleep Diary data and were analyzed
Exercise training improved sleep initiation and continuity in GAD patients
Improvements were larger for resistance exercise and during the weekend
Acknowledgments
This research was supported by an internal grant from The University of Georgia’s College of Education. Support for C.E. Kline was provided by NIH grant K23 HL118318.
Footnotes
Author Contributions
M. P. Herring and P. J. O’Connor conceptualized the trial, developed the trial design, and contributed to data collection. M. P. Herring performed data processing and analysis. M. P. Herring, C. E. Kline, and P. J. O’Connor contributed to interpretation of results. M. P. Herring drafted the initial manuscript and C. E. Kline and P. J. O’Connor provided critical revisions. All authors approved the final version of the manuscript for submission. M. P. Herring is the primary and corresponding author and is responsible for its content.
The authors report no conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew P. Herring, Email: matthew.herring@ul.ie.
Christopher E. Kline, Email: klinec@upmc.edu.
Patrick J. O’Connor, Email: poconnor@uga.edu.
References
- Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a sevenday recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological psychiatry. 1996;39(6):411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. American Journal of Public Health. 1997;87(10):1649–1653. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Psychometric properties of the Penn State Worry Questionnaire in a clinical anxiety disorders sample. Behaviour research and therapy. 1992;30(1):33–37. doi: 10.1016/0005-7967(92)90093-v. [DOI] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, Barlow DH. Anxiety disorders interview schedule for DSM-IV: Adult version. Oxford University Press; 1994. [Google Scholar]
- Buboltz W, Jenkins SM, Soper B, Woller K, Johnson P, Faes T. Sleep habits and patterns of college students: an expanded study. Journal of College Counseling. 2009;12(2):113–124. [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultney JF. Weekend-weeknight shifts in sleep duration predict risk for metabolic syndrome. Age. 2014;48(6.51):32–67. [Google Scholar]
- Hart CN, LaRose JG, Fava JL, James BL, Wing RR. The association between time in bed and obesity risk in young adults. Behavioral sleep medicine. 2013;11(5):321–327. doi: 10.1080/15402002.2012.700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Olkin I, Statistiker M, Olkin I, Olkin I. Statistical methods for meta-analysis. Academic Press; New York: 1985. [Google Scholar]
- Herring MP, Jacob ML, Suveg C, Dishman RK, O’Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- Herring MP, Jacob ML, Suveg C, O’Connor PJ. Effects of short-term exercise training on signs and symptoms of generalized anxiety disorder. Mental Health and Physical Activity. 2011;4(2):71–77. [Google Scholar]
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of Exercise Training on Sleep Apnea: A Meta-analysis. Lung. 2014;192(1):175–184. doi: 10.1007/s00408-013-9511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of psychosomatic research. 2008;64(4):443–449. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. Journal of psychiatric research. 2006;40(8):700–708. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kim CW, Choi MK, Im HJ, Kim OH, Lee HJ, Song J, Park KH. Weekend catch-up sleep is associated with decreased risk of being overweight among fifth-grade students with short sleep duration. Journal of sleep research. 2012;21(5):546–551. doi: 10.1111/j.1365-2869.2012.01013.x. [DOI] [PubMed] [Google Scholar]
- King AC, Pruitt LA, Woo S, Castro CM, Ahn DK, Vitiello MV, Bliwise DL. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(9):997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, Youngstedt SD. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Irish LA, Krafty RT, Sternfeld B, Kravitz HM, Buysse DJ, Hall MH. Consistently High Sports/Exercise Activity Is Associated with Better Sleep Quality, Continuity and Depth in Midlife Women: The SWAN Sleep Study. Sleep. 2013;36(9):1279–1288. doi: 10.5665/Sleep.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Sui X, Hall MH, Youngstedt SD, Blair SN, Earnest CP, Church TS. Dose–response effects of exercise training on the subjective sleep quality of postmenopausal women: exploratory analyses of a randomised controlled trial. BMJ open. 2012;2(4):e001044. doi: 10.1136/bmjopen-2012-001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Annals of epidemiology. 2013;23(6):361–370. doi: 10.1016/j.annepidem.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep medicine. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS. Survey Questions About Sleep Duration: Does Asking Separately About Weekdays and Weekends Matter? Behavioral sleep medicine. 2014;12(2):158–168. doi: 10.1080/15402002.2013.778201. [DOI] [PubMed] [Google Scholar]
- Lineberger M, Carney C, Edinger J, Means M. Defining insomnia: quantitative criteria for insomnia severity and frequency. Sleep. 2006;29(4):479–485. doi: 10.1093/sleep/29.4.479. [DOI] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal of Adolescent Health. 2010;46(2):124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- McNair DM, Droppleman LF, Lorr M. Edits manual for the profile of mood states: POMS: Edits. 1992. [Google Scholar]
- Monk TH, REYNOLDS CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, Ritenour AM. The Pittsburgh sleep diary. Journal of sleep research. 1994;3(2):111–120. [PubMed] [Google Scholar]
- Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Medicine Reviews. 2000;4(3):263–276. doi: 10.1053/smrv.1999.0096. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of psychiatric research. 2003;37(1):9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Kerkhofs M, Kempenaers C, Mendlewicz J. EEG sleep studies in patients with generalized anxiety disorder. Psychiatry research. 1988;26(2):183–190. doi: 10.1016/0165-1781(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. International Review of Psychiatry. 2005;17(4):229–236. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- Passos GS, Poyares D, Santana MG, D’Aurea CVR, Youngstedt SD, Tufik S, de Mello MT. Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep medicine. 2011;12(10):1018–1027. doi: 10.1016/j.sleep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Passos GS, Poyares D, Santana MG, Garbuio SA, Tufik S, Mello MT. Effect of acute physical exercise on patients with chronic primary insomnia. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2010;6(3):270. [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Zee J, Swinkels C, Kloss J, Morgan K, David B, Morales K. The incidence and temporal patterning of insomnia: a second study. Journal of sleep research. 2014 doi: 10.1111/jsr.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M, Kinrys G, Krystal A, McCall WV, Roth T, Schaefer K, Krishnan R. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Archives of general psychiatry. 2008;65(5):551. doi: 10.1001/archpsyc.65.5.551. [DOI] [PubMed] [Google Scholar]
- Ramsawh HJ, Stein MB, Belik SL, Jacobi F, Sareen J. Relationship of anxiety disorders, sleep quality, and functional impairment in a community sample. Journal of psychiatric research. 2009;43(10):926–933. doi: 10.1016/j.jpsychires.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Schweitzer P. Principles and practice of sleep medicine. 3. Philadelphia: WB Saunders; 2000. Drugs that disturb sleep and wakefulness; pp. 441–461. [Google Scholar]
- Sekine M, Tatsuse T, Cable N, Chandola T, Marmot M. U-shaped associations between time in bed and the physical and mental functioning of Japanese civil servants: the roles of work, family, behavioral and sleep quality characteristics. Sleep medicine. 2014 doi: 10.1016/j.sleep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch R. State-Trait Anxiety Inventory for Adults: Manual and Sample: Manual, Instrument and Scoring Guide. Consulting Psychologists Press; 1983. [Google Scholar]
- Stea T, Knutsen T, Torstveit M. Association between short time in bed, health-risk behaviors and poor academic achievement among Norwegian adolescents. Sleep medicine. 2014;15(6):666–671. doi: 10.1016/j.sleep.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Guthrie KA, Ensrud KE, LaCroix AZ, Larson JC, Dunn AL, Newton KM. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014;21(4):330–338. doi: 10.1097/GME.0b013e31829e4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MR, Stevens D, Faulkner GE. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Preventive medicine. 2013;56(2):112–117. doi: 10.1016/j.ypmed.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Tsai LL, Li SP. Sleep patterns in college students: Gender and grade differences. Journal of psychosomatic research. 2004;56(2):231–237. doi: 10.1016/S0022-3999(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Journal of physiotherapy. 2012;58(3):157–163. doi: 10.1016/S1836-9553(12)70106-6. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD. Effects of exercise on sleep. Clinics in sports medicine. 2005;24(2):355–365. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kline CE. Epidemiology of exercise and sleep*. Sleep and Biological Rhythms. 2006;4(3):215–221. doi: 10.1111/j.1479-8425.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Archives of general psychiatry. 2001;58(8):787. doi: 10.1001/archpsyc.58.8.787. [DOI] [PubMed] [Google Scholar]