Abstract
The clinical significance of pancreatic intraepithelial neoplasia (PanIN) lesions in non-neoplastic pancreata of pancreatic ductal adenocarcinoma (PDAC) patients remains controversial. As chronic inflammation has been recently demonstrated to promote dissemination of in situ precancerous lesions, we investigated the prognostic significance of PanINs associated with chronic pancreatitis (CP) in PDAC patients. This retrospective study analyzed 125 curatively resected PDAC specimens for the presence of PanIN and CP. Univariate and multivariate analyses were performed to identify significant predictive factors for poor disease-free survival (DFS) and overall survival (OS). Immunohistochemical staining for E-cadherin and S100A4, markers of epithelial-mesenchymal transition, was performed on resected specimens containing PanIN-3 lesions. CP was observed in 27.2% (34/125) and PanIN-3 in 25.6% (32/125) of specimens. In the presence of CP, PanIN-3 was significantly associated with decreased survival (DFS: 4.3 vs 15.5 months, P = 0.021; OS: 16.3 vs 30.9 months, P = 0.004). PanIN-3 was not a prognostic factor in the absence of CP. The presence of both PanIN-3 and CP was associated with a reduced survival compared to the other cases, in both univariate (DFS: P = 0.039; OS: P = 0.023) and multivariate (DFS: P = 0.020; OS: P = 0.076) analyses. Furthermore, E-cadherin loss and S100A4 expression were more frequently observed in PanIN-3 lesions of CP specimens than in those of non-CP specimens, although not statistically significant. PanIN-3 in association with CP is a significant prognostic factor for decreased survival in PDAC patients, suggesting that chronic inflammation may accelerate the progression of preinvasive high-grade PanIN.
Keywords: Chronic pancreatitis, inflammation, pancreatic cancer, pancreatic intraepithelial lesions, prognosis
At diagnosis, only 10–20% of patients with pancreatic cancer are eligible for curative surgery.1,2 Furthermore, post-surgical local recurrences or metastases are not infrequent.3,4 While pancreatic intraepithelial neoplasia (PanIN; graded 1–3, depending on the degree of cytologic atypia) are well-established precursor lesions of pancreatic ductal adenocarcinoma (PDAC),5 their prognostic significance in non-cancerous pancreata remains controversial. Numerous clinicopathological studies on PDAC have identified prognostic factors associated with the primary tumor, such as size, extent of invasion, and lymphovascular and perineural invasion. The presence of high-grade PanIN in resected specimens was not found to influence survival, even if present at the pancreatic transection margin.6 As PDAC is a rapidly progressive cancer, it is possible that the PDAC itself might be the primary determinant of prognosis and not the co-existing preinvasive lesion, which may take longer to become an invasive carcinoma.
Inflammation has been demonstrated to play a crucial role in all stages of tumor development including tumor initiation, promotion, malignant transformation, invasion, and even metastasis. Chronic inflammation, such as obesity or chronic pancreatitis (CP), for example, is known to predispose patients to PDACs.7,8 Interestingly, even non-invasive PanIN-3 cells have been shown to circulate and metastasize to the liver under inflammatory conditions.9 Such observations raise the question of whether PanIN may also be involved in tumor recurrence in PDAC patients. Therefore, we aimed to investigate whether the presence of PanIN in completely resected (R0) PDAC specimens conferred any prognostic value depending on the presence or absence of CP.
Methods
Patients and pathological examination
This retrospective study enrolled 125 subjects who underwent R0 resection for PDAC at Seoul National University Bundang Hospital between May 2003 and March 2013. Subjects who did not undergo R0 resection, were positive for PanIN-3 at the pancreatic transection margins, who received neoadjuvant treatment, or died within 30 days of surgery were excluded. The clinicopathological parameters investigated included age, sex, type of surgery, tumor size, lymph node status and histological differentiation, presence of angiolymphatic invasion, venous invasion, perineural invasion, presence and grade of PanIN, and presence of underlying CP. The size and extent of the primary tumor and the presence of nodal metastasis were classified according to the 7th AJCC staging manual.10 Effective adjuvant therapy was defined as a completion of at least one cycle of chemotherapy or a planned radiation dose delivery.
The definition and classification of PanIN were in accordance with the international consensus guidelines.5 Briefly, PanIN-1 was defined by a flat or papillary epithelial lesion composed of columnar cells with basally located nuclei and abundant supranuclear mucin. PanIN-2 lesions had slightly more complex architecture, with more nuclear changes, including some loss of polarity, nuclear crowding, enlarged nuclei, pseudostratification, and hyperchromasia. Lesions with substantial architectural and cytologic atypia were designated as PanIN-3. In cases where multiple degrees of PanIN were detected in the same sample, the highest degree of PanIN was recorded. The presence of a localized area of chronic inflammation and fibrosis adjacent to (<5 mm from) the cancer lesion was not counted as CP, in order to exclude the possibility of peritumoral stromal reaction; only the presence of inflammation and fibrosis in the distant parenchyme (≥5 mm from the cancer) was regarded as CP for this study. Cases with CP demonstrated variable degrees of lobular atrophy with loss of acinar cells and fibrosis, retention of the islets of Langerhans, some ectatic pancreatic ducts and mild stromal lymphocytic infiltration.
Immunohistochemical analysis of E-cadherin and S100A4 expression
Immunohistochemistry was performed to evaluate E-cadherin and S100A4 expression status in PanIN-3 and PDACs. Briefly, 4 μm-thick tissue sections were obtained from archival formalin-fixed paraffin-embedded tissues, deparaffinized in xylene, rehydrated in graded alcohol and quenched in 3% H2O2/methanol. Antigen retrieval was performed by microwave heating in citrate buffer (pH 6.0), and tissue sections were incubated with anti-E-cadherin antibody (1:150; Zymed, San Francisco, CA, USA) or anti-S100A4 antibody (1:1500; Dako, Glostrup, Denmark) at room temperature for 30 min. After rinsing, sections were treated with a secondary antibody (EnVision Rabbit/Mouse kit; DAKO), stained with 3,3-diaminobenzidine (DAKO), and counterstained with hematoxylin. E-cadherin expression was scored as follows: intact, complete membrane staining; partial loss, loss of membrane expression in <50% of PanIN or PDAC cells; and complete loss, loss of membrane expression in ≥50% of PanIN or PDAC cells. Both partial loss and complete loss were regarded as loss of E-cadherin for statistical purposes. S100A4 expression was scored as positive when at least focal (more than 5%) nucleocytoplasmic expression was seen in the PanIN or PDAC cells. The same scoring scheme was used regardless of the number of PanIN lesions present on the same immunohistochemistry slide.
Statistical analysis
Overall survival (OS), defined as the period from pancreatic surgery until the day of death by any cause, was the primary endpoint. Patients surviving to the final follow-up session, and who did not visit the hospital thereafter, were regarded as censored. Disease-free survival (DFS) was calculated from the day of surgery to the first day of recurrence during regular follow-up.
The Pearson’s χ2 and Fisher’s exact tests were used to determine differences between categorical groups. The relationship between PanIN and CP was investigated by linear by linear association. Univariate analyses for DFS and OS according to the clinicopathologic variables such as the presence and grade of PanIN in resected tumors, underlying CP, tumor size, nodal status, other pathological findings, and the use of adjuvant therapy performed by the Kaplan–Meier method, log-rank tests and Cox proportional hazards regression method. Clinicopathological variables reaching P < 0.1 in univariate analysis were entered into multivariate models for DFS and OS. Separate survival analyses were performed in the CP and non-CP groups to investigate the prognostic significance of PanIN-3. The SPSS software (version 21; IBM Corporation, Armonk, NY, USA) was used for statistical analyses, and all tests were two-sided. P-values < 0.05 were considered statistically significant.
Results
Baseline patient characteristics
The clinicopathological characteristics of all 125 cases are presented in Table1. Mean age was 63 years (range, 40–81 years). Most had T3 lesions (97%), and 76 patients (61%) were classified as pN1. Patients had undergone pancreaticoduodenectomy (n = 83), distal pancreatectomy (n = 32), or subtotal or total pancreatectomy (n = 10). Adjuvant therapy was given in 36 cases (28.8%). Angiolymphatic, venous, and perineural invasion was noted in 47%, 34%, and 86%, respectively. PanIN was present in 57% of specimens (18 PanIN-1, 21 PanIN-2 and 32 PanIN-3). CP was observed in the non-neoplastic pancreatic parenchyma in 34 cases (27%). Diffuse interlobular fibrosis, acinar atrophy, islet cell hyperplasia, and chronic inflammatory cell infiltration were also observed.
Table 1.
Baseline patient characteristics
| Characteristics | All patients (n = 125) | PanIN3 with CP (n = 12) | Non-PanIN3 with CP (n = 113) | P-value |
|---|---|---|---|---|
| Age, years; mean ± SD | 63 ± 8.7 | 66 ± 8.2 | 63 ± 8.7 | 0.277 |
| Male, n (%) | 69 (55.2) | 6 (50.0) | 63 (55.8) | 0.703 |
| T classification, n (%) | ||||
| T1 | 1 (0.8) | 0 (0) | 1 (0.9) | 0.141 |
| T2 | 2 (1.6) | 1 (8.3) | 1 (0.9) | |
| T3 | 122 (97.6) | 11 (91.7) | 111 (98.2) | |
| N classification, n (%) | ||||
| N0 | 49 (39.2) | 3 (25.0) | 46 (40.7) | 0.230 |
| N1 | 76 (60.8) | 9 (75.0) | 67 (59.3) | |
| Surgery type, n (%) | ||||
| Pancreaticoduodenectomy | 83 (66.4) | 9 (75.0) | 74 (65.5) | 0.600 |
| Distal pancreatectomy | 32 (25.6) | 2 (16.7) | 30 (36.5) | |
| Subtotal pancreatectomy | 5 (4.0) | 5 (4.4) | 5 (4.4) | |
| Total pancreatectomy | 5 (4.0) | 4 (3.5) | 4 (3.5) | |
| Main location of tumor, n (%) | ||||
| Head | 87 (69.6) | 10 (83.3) | 77 (68.1) | 0.715 |
| Body | 18 (14.4) | 1 (8.3) | 17 (15.0) | |
| Tail | 20 (16.0) | 1 (8.3) | 19 (16.8) | |
| Histologic classification, n (%) | ||||
| WD | 7 (5.6) | 2 (16.7) | 5 (4.4) | 0.337 |
| MD | 101 (80.8) | 9 (75.0) | 92 (81.4) | |
| PD | 14 (11.2) | 1 (8.3) | 13 (11.5) | |
| UD | 3 (2.4) | 0 (0.0) | 3 (2.7) | |
| AL invasion, n (%) | 59 (47.2) | 8 (66.7) | 51 (45.1) | 0.155 |
| VE invasion, n (%) | 43 (33.6) | 6 (50.0) | 36 (31.9) | 0.172 |
| PN invasion, n (%) | 107 (85.6) | 10 (83.3) | 97 (85.8) | 0.543 |
| Adjuvant Therapy, n (%) | 36 (28.8) | 1 (8.3) | 35 (31.0) | 0.088 |
| PanIN grade, n (%) | ||||
| No PanIN | 54 (43.2) | |||
| PanIN-1 | 18 (14.4) | |||
| PanIN-2 | 21 (16.8) | |||
| PanIN-3 | 32 (25.6) | |||
| CP, n (%) | 34 (27.2) | |||
AL, angiolymphatic; CP, chronic pancreatitis; MD, moderately differentiated; PanIN, pancreatic intraepithelial neoplasia; PD, poorly differentiated; PN, perineural; UD, undifferentiated; VE, venous; WD, well differentiated.
PanIN and CP
Subjects were divided into CP and non-CP groups, according to the presence or absence of CP in the non-neoplastic parenchyma. PanIN was more commonly detected in the former, with marginal significance (72% vs 51%, P = 0.052). Furthermore, CP was more commonly observed in higher PanIN grades (no PanIN: 18%, PanIN-1: 27%, PanIN-2: 33%, PanIN-3: 38%; P = 0.044). The clinicopathological features of the patients were compared according to the presence of PanIN3 and CP (Table1). There were no significant differences in terms of clinicopathological features between PDAC specimens harboring both PanIN3 and CP (n = 12), and the remaining cases (n = 113).
Decreased DFS and OS in cases with concomitant PanIN-3 and CP
During a median follow-up period of 22 months, 78 patients (62.4%) experienced recurrence of PDAC after resection; median DFS was 10.9 months. Univariate analysis revealed no significant difference in DFS between patients with and without PanIN-3 (11.6 vs 10.9 months, P = 0.808; Fig.1a). Similarly, no significant difference in OS was found between patients with and without PanIN-3 (19.5 vs 20.6 months, P = 0.311; Fig.1b). Clinicopathological variables associated with decreased DFS included higher N stage (N1; P = 0.015), perineural invasion (P = 0.044), and angiolymphatic invasion (P = 0.056). N1 stage (P = 0.054), angiolymphatic invasion (P = 0.098), and venous invasion (P = 0.063) were marginally significant for decreased OS. And adjuvant therapy was significant factor in both DFS (P = 0.050) and OS (P = 0.010).
Figure 1.
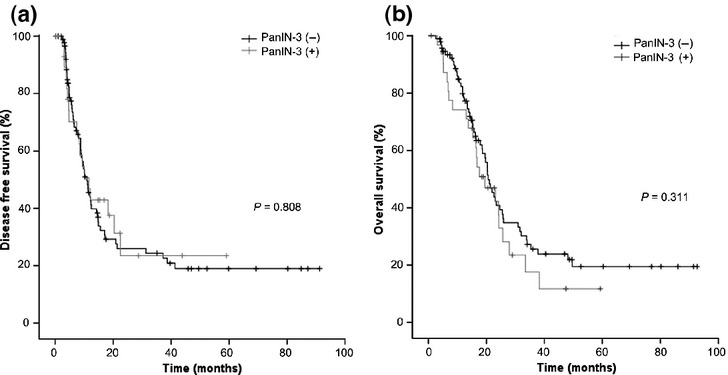
No association between PanIN-3 in resected tumor samples and (a) disease-free survival (P = 0.808) or (b) overall survival (P = 0.311).
We performed a subgroup analysis of CP (n = 34) and non-CP (n = 91) groups, to analyze the differences in DFS and OS in relation to the presence of PanIN-3. In the non-CP group, there were no significant differences in DFS and OS in relation to the presence of PanIN-3 (18.1 vs 9.6 months, P = 0.130 and 24.1 vs 20.3 months, P = 0.785, respectively; data not shown). In the CP group, however, the presence of PanIN-3 was associated with a significant decrease in both DFS (4.3 vs 15.5 months, P = 0.021; Fig.2a) and OS (16.3 vs 30.9 months, P = 0.004; Fig.2b).
Figure 2.
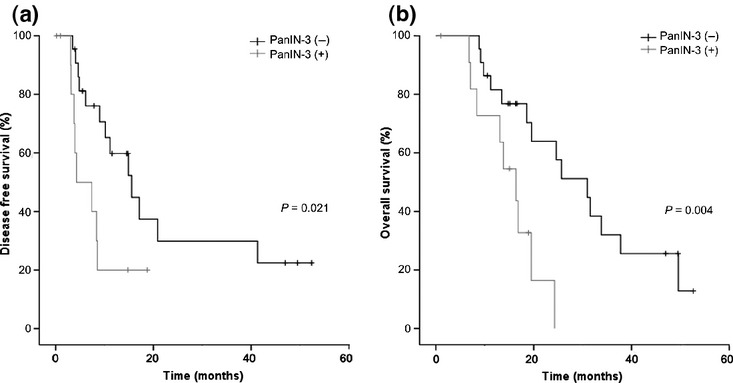
Association between PanIN-3 in resected tumor samples and (a) disease-free survival (P = 0.021), and (b) overall survival (P = 0.004), in chronic pancreatitis (CP) patient subgroup.
Univariate analysis of DFS using Cox proportional hazards regression showed that the combined presence of PanIN-3 and CP was associated with poor prognosis compared to other PDAC cases (hazard ratio [HR]: 2.17; 95% confidence interval [CI]: 1.04–4.56; P = 0.039). After adjustments for node status, angiolymphatic invasion, perineural invasion, and adjuvant therapy, multivariate analysis confirmed concomitant PanIN-3 and CP as a significant determinant of DFS (HR: 2.51; 95% CI: 1.15–5.46; P = 0.020; Table2). Concomitant PanIN-3 and CP was also significantly associated with poor OS in univariate analysis (HR: 2.23; 95% CI: 1.36–6.30; P = 0.023). On multivariate analysis after adjusting for node status, angiolymphatic invasion, adjuvant therapy and venous invasion, the presence of both PanIN3 and CP was still associated with a decreased OS, although marginally significant (HR: 1.93; 95% CI: 0.93-3.98; P = 0.076; Table3). In patients with low-grade PanIN (1 and 2), the presence or absence of CP did not influence prognosis (HR: 2.38; 95% CI: 0.87–6.54; P = 0.091 for DFS; and HR: 1.83; 95% CI: 0.65–5.16, P = 0.248 for OS; data not shown).
Table 2.
Cox proportional hazards regression analysis of disease-free survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (≥70 years) | 1.03 (0.62–1.71) | 0.913 | ||
| Sex (Male) | 1.35 (0.83–2.04) | 0.244 | ||
| CA 19-9 (≥1000 U/mL) | 1.56 (0.91–2.68) | 0.104 | ||
| T stage (T3/T2,T1) | 3.2 (0.44–23.04) | 0.248 | ||
| N stage (N1/N0) | 1.81 (1.12–2.91) | 0.015 | 1.98 (1.21–3.22) | 0.006 |
| Differentiation (UD,PD/MD,WD) | 1.07 (0.53–2.15) | 0.849 | ||
| AL invasion | 1.55 (0.99–2.42) | 0.056 | ||
| VE invasion | 1.43 (0.91–2.28) | 0.124 | ||
| PN invasion | 2.12 (1.02–4.43) | 0.044 | 2.23 (1.06–4.72) | 0.036 |
| CP | 0.99 (0.60–1.64) | 0.987 | ||
| PanIN-3 | 0.93 (0.55–1.59) | 0.808 | ||
| Adjuvant therapy | 0.62 (0.39–1.00) | 0.050 | 0.62 (0.38–1.02) | 0.058 |
| PanIN-3 with CP | 2.17 (1.04–4.56) | 0.039 | 2.51 (1.15–5.46) | 0.020 |
AL, angiolymphatic; CI, confidential interval; CP, chronic pancreatitis; HR, hazard ratio; MD, moderately differentiated; PanIN, pancreatic intraepithelial neoplasia PD, poorly differentiated; PN, perineural; UD, undifferentiated; VE, venous; WD, well differentiated.
Table 3.
Cox proportional hazards regression analysis of overall survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (≥70 years) | 1.18 (0.72–1.94) | 0.516 | ||
| Sex (Male) | 1.23 (0.79–1.91) | 0.370 | ||
| CA 19-9 (≥1000 U/mL) | 1.17 (0.67–2.06) | 0.584 | ||
| T stage (T3/T1,2) | 2.57 (0.36–18.50) | 0.348 | ||
| N stage (N1/N0) | 1.59 (0.99–2.54) | 0.054 | ||
| Differentiation (UD, PD/MD, WD) | 1.62 (0.87–3.02) | 0.125 | ||
| AL invasion | 1.45 (0.93–2.26) | 0.098 | ||
| VE invasion | 1.53 (0.98–2.41) | 0.063 | ||
| PN invasion | 1.71 (0.82–3.56) | 0.151 | ||
| CP | 1.06 (0.65–1.72) | 0.819 | ||
| PanIN-3 | 1.28 (0.79–2.09) | 0.313 | ||
| Adjuvant therapy | 0.53 (0.32–0.86) | 0.010 | 0.56 (0.34–0.92) | 0.021 |
| PanIN-3 with CP | 2.23 (1.12–4.69) | 0.023 | 1.93 (0.93–3.98) | 0.076 |
More frequent E-cadherin expression loss and S100A4 expression in PanIN-3 associated with CP
Expression of E-cadherin and S100A4 (markers of epithelial-mesenchymal transition; EMT) was evaluated in 28 specimens with PanIN-3 lesions: eight with CP and 20 without. Although not statistically significant, E-cadherin loss and S100A4 expression were more frequently observed in PanIN-3 lesions of CP specimens than in those of non-CP specimens (E-cadherin: 87.5% vs 65.0%; S100A4: 62.5% vs 40.0%, respectively) (Fig.3). In order to explore the relationship between EMT-related marker expression status in PanINs and PDACs, we also performed immunohistochemical scoring for S100A and E-cadherin on the PDAC lesions for the same 28 cases that contained PanIN3 lesions. We found S100A4 expression and E-cadherin loss in 26 (92.9%) and 27 (96.4%) PDACs, respectively, and all cases where PanINs expressed S100A4 or showed loss of E-cadherin demonstrated the same findings in the corresponding cancer lesions. In reverse, S100A4 expression and E-cadherin loss were seen in 10 and 8 PDACs, respectively, in which the concurrent PanIN3 lesions did not express these markers.
Figure 3.
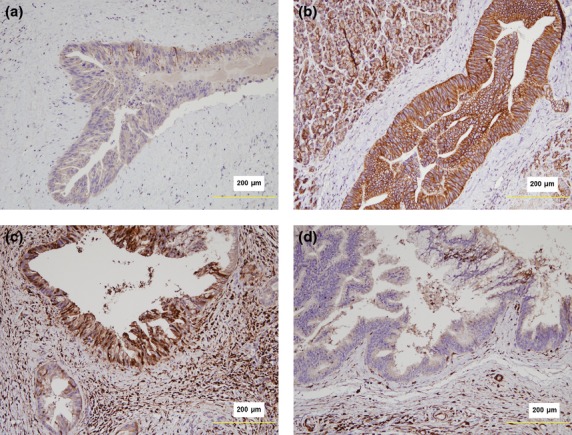
(a) Loss of E-cadherin expression in PanIN-3 lesions associated with chronic pancreatitis (CP). (b) Intact E-cadherin expression in PanIN-3 lesions with no background of CP. (c) Strong nucleocytoplasmic expression of S100A4 in a PanIN-3 lesion associated with CP. (d) No S100A4 expression in a PanIN-3 lesion without associated CP.
Discussion
In this study, we hypothesized that CP in the non-neoplastic pancreas may provide soil in which preinvasive PanIN-3 cells may undergo EMT and subsequent dissemination, and that the presence of PanIN-3 may be a negative prognostic marker in PDAC patients with CP. Although we do not provide direct experimental evidence for this postulated sequence of events, we have demonstrated that the combined presence of PanIN-3 and CP in surgically resected PDAC specimens is associated with decreased DFS and OS. Conversely, PanIN-3 status did not correlate with survival in the absence of CP.
A recent study showed that the presence of PanIN-3 at the resected margin did not influence survival in patients who underwent R0 resection for pancreatic cancer,11 and whether further pancreatectomy should be performed in patients with PanIN-3 at the surgical transection margin, at the expense of complications such as endocrine insufficiency, is still debated.12 The prognostic significance of PanIN in PDAC patients with CP has not been reported before, and the results of this study suggest that the concurrence of PanIN-3 and CP could be of prognostic value in PDAC, in addition to other markers such as stage, lymphovascular invasion, adjuvant therapy and perineural invasion.
Recent studies have shown that even early stage cancer cells such as carcinoma in situ had the capacity to metastasize in breast cancer patients before the development of an overt malignancy.13–15 In addition, using a pancreatic cancer mouse model generated by the inactivation of p16Ink4a and p19Arf, Rhim and his colleagues demonstrated that experimental pancreatitis induced by chemical or surgical methods increased circulating pancreatic cells in the bloodstream, and identified seeding of PanIN-3 cells to the liver under pancreatitis.9 Conversely, the number of circulating pancreatic cells was decreased when inflammation was suppressed by steroid. Based on these findings, it was suggested that dissemination of pancreatic cells could occur even before the development of an overt malignancy, and that inflammation had a crucial role in pancreatic cancer cell dissemination. Furthermore, a recent human study identified circulating pancreatic epithelial cells in subjects with precancerous pancreatic cystic lesions, supporting early hematogenous tumor dissemination.16 Chronic inflammation may also play a role in facilitating or accelerating tumorigenesis, through additional genetic events such as tumor suppressor gene mutation and oncogenic mutations. Indeed, chronic inflammation has been closely linked with preneoplastic lesions, for example, dysplasia-associated lesions in chronic ulcerative colitis.17–19 A similar relationship between chronic inflammation (chronic pancreatitis) and dysplasia (PanIN) could also be suggested in the pancreas from our data.
Epithelial-mesenchymal transition is an essential biologic process for tumor progression. EMT was found to be abundant in inflammatory foci of the pancreas, suggesting that inflammation may promote EMT and early dissemination of PanIN-3.9 Expression of S100A4 protein and loss of membranous E-cadherin expression are markers of EMT. We have previously reported that expression of EMT-related markers in PDAC was associated with poor histologic differentiation, more frequent lymphatic, vascular and perineural invasion and higher tumor stage. In addition, S100A4 expression in pancreatic cancers was significantly associated with earlier tumor recurrence.20 Although statistical significance was not reached in this study (possibly due to the small number of cases studied), E-cadherin expression loss and S100A4 protein expression were more frequently seen in PanIN-3 lesions associated with CP compared to those without a CP background, supporting the notion that chronic inflammation may contribute to EMT and offering potential clinical target for prevention of metastasis by inhibition of EMT.
While the presence of PanIN-3 lesions in a background of CP showed prognostic significance in our cohort of PDAC patients, the presence of lower-grade PanIN (1 and 2) was not, irrespective of inflammatory status. It is well known that oncogenic mutations usually occur during tumor initiation or the earlier stages of tumor progression (PanIN-1 or –2), while mutation of tumor suppressor genes is a later phenomenon (PanIN-3 or overt carcinoma) during pancreatic tumorigenesis.21,22 There is also experimental evidence that circulating cells from murine PanINs with only KRAS mutation were not competent enough to form colonies under experimental pancreatitis, while those with additional p53 loss had clonal growth properties.9,23
It is too speculative at this point to suggest EMT of intraepithelial precursor lesions as a mechanism for the poor prognosis of PDAC, especially as the number of cases subjected to immunohistochemical stains for EMT markers was small, and we did not see statistically significant associations between EMT-marker expression status and CP. EMT of the cancer cells would be more likely to contribute to the aggressive behavior of PDAC. On examining the EMT-marker expression status of PDACs, we found S100A4 expression and E-cadherin loss in the majority of PDACs, and all cases where PanINs expressed S100A4 or showed loss of E-cadherin demonstrated the same findings in the corresponding cancer lesions. In reverse, S100A4 expression and E-cadherin loss were seen in 10 and eight PDACs, respectively, in which the concurrent PanIN3 lesions did not express these markers. Although hard to generalize due to the limited number of cases studied, this may imply that EMT features may be increasingly acquired during progression of PanIN3 lesions to invasive carcinomas. Nevertheless, the association between concurrent PanIN3 and CP with a poor prognosis – even after adjusting for variables related to the cancer, such as adjuvant therapy, angiolymphatic and venous invasion and nodal status – is an interesting finding, and suggests a prognostic significance for PanIN3 in curatively resected PDAC cases, independent of clinicopathological variables related to the cancer itself.
There are several limitations to the current study. First, this study was conducted retrospectively. However, data loss was minimized through the use of a prospectively maintained database extracted from the electronic medical records system. Secondly, examination of the remaining pancreatic tissue would have been important to more accurately assess the prognostic value of concomitant PanIN-3 and CP, although this was not feasible. Finally, the number of patients with concomitant PanIN-3 and CP was relatively small. Evaluation of the long-term prognostic impact will require a large-scale prospective study.
In conclusion, PanIN-3 lesions in the presence of CP were associated with poor prognosis in patients who underwent curative surgery for PDAC and were more frequently associated with S100A4 expression and loss of E-cadherin expression. The presence of chronic inflammation may enhance the dissemination of preinvasive high-grade PanIN cells, possibly through effects on EMT.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–31. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- Sergeant G, Ectors N, Van Steenbergen W, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–4. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–8. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- Matthaei H, Hong SM, Mayo SC, et al. Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer. Ann Surg Oncol. 2011;18:3493–9. doi: 10.1245/s10434-011-1745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Vijayalekshmi R, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Matthaei H, Hong S-M, Mayo SC, et al. Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer. Indian J Surg Oncol. 2011;2:9–15. doi: 10.1007/s13193-011-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–47. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Du Y-CN, Jechlinger M, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–4. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüsemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–51. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik FD, Mooiweer E, van der Have M, et al. Adenomas in patients with inflammatory bowel disease are associated with an increased risk of advanced neoplasia. Inflamm Bowel Dis. 2013;19:342–9. doi: 10.1097/MIB.0b013e318286f771. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim H, Hwang J-H, et al. CD24 and S100A4 expression in resectable pancreatic cancers with earlier disease recurrence and poor survival. Pancreas. 2014;43:380–8. doi: 10.1097/MPA.0000000000000097. [DOI] [PubMed] [Google Scholar]
- Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–6. [PubMed] [Google Scholar]
- Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–12. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


