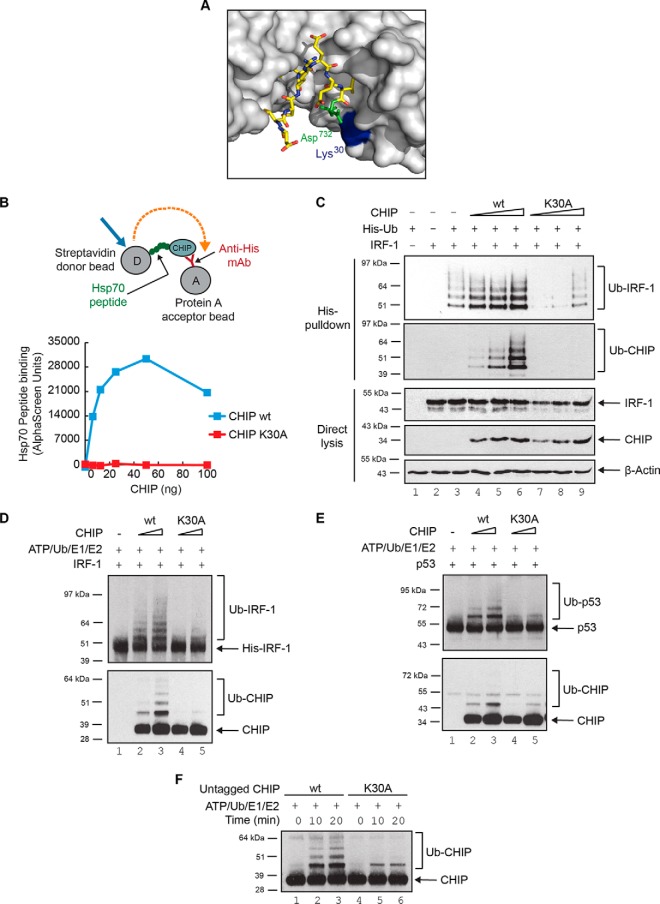
Fig. 2.
CHIP-K30A is intrinsically defective in E3-ligase activity. (A) Close-up of the Hsp90 binding site on CHIP extracted from the crystal structure of mCHIP dimer (protomers in shades of gray; also see Fig 1D) in complex with Hsp90 peptide (yellow sticks; PDB code 2C2L) generated using PyMOL v1.4.1. Lys30 on CHIP and Asp732 on Hsp90 are highlighted in blue and green respectively. (B) An AlphaScreen assay was set up (see cartoon) to measure binding dynamics of His-CHIP wt or K30A mutant with biotin-tagged Hsp70 peptide (GPTIEEVD) in solution. (C) Ubiquitination of exogenous IRF-1 in H1299 cells transiently transfected with plasmids encoding CHIP wt or K30A mutant and His-tagged ubiquitin. Immunoblots show ubiquitinated protein (His-pulldown) and total protein (Direct lysis). (D, E) In vitro ubiquitination assays were assembled using ATP, ubiquitin, UBE1, UbcH5a, untagged CHIP wt or K30A, and His-IRF-1 (D) or untagged p53 (E) as substrate. Reactions were analyzed by 4–12% NuPAGE/immunoblot. (F) Immunoblot of in vitro ubiquitination assays assembled as above except in the absence of substrate to study auto-ubiquitination of untagged CHIP wt or K30A proteins over time.