Figure 1.
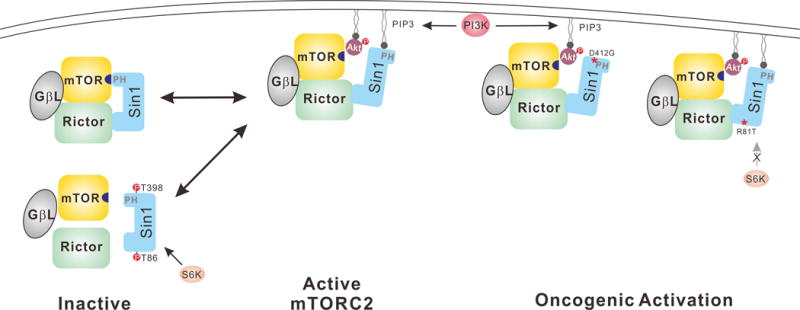
Dual role of Sin1 in mTORC2 regulation. In the absence of stimulation, the PH domain of Sin1 binds to and suppresses mTORC2 activity. Upon PI3K activation, the increased PtdIns(3,4,5)P3 binds to Sin1 PH domain, leading to membrane recruitment as well as relieve of inhibition of mTORC2 by Sin1. The active mTORC2 phosphorylates and activates the membrane associated Akt. Sin1 can be phosphorylated by S6K and Akt to constitutive a negative regulation. Cancer associated mutations in Sin1 can potentiate mTORC1 activity by disrupting the inhibitory phosphorylation in Sin1 or interaction with mTOR.
