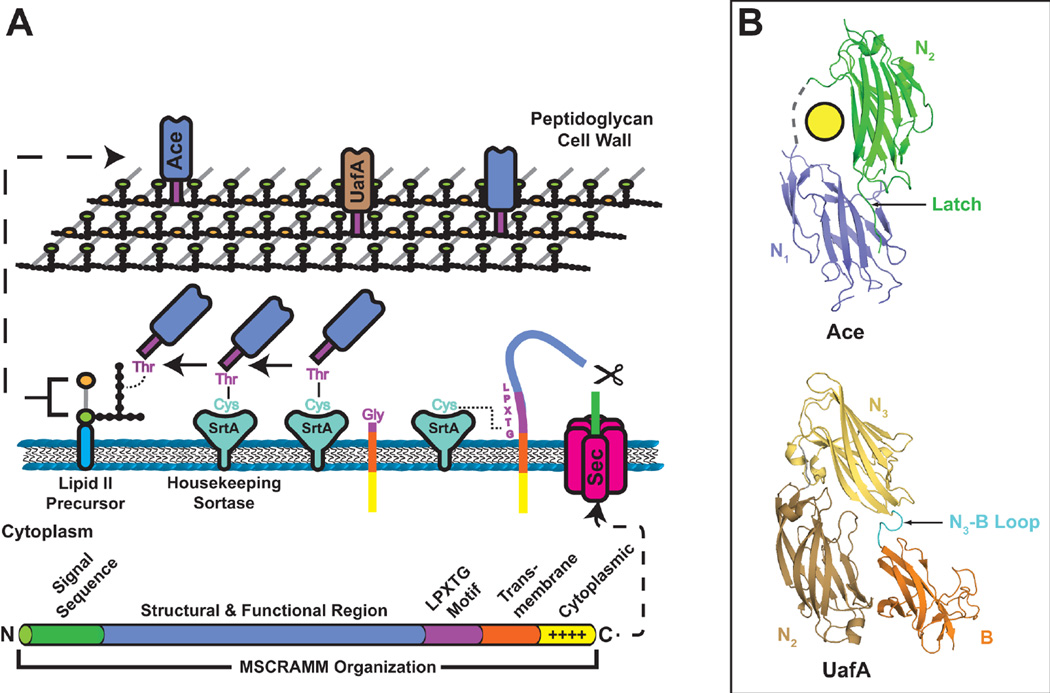
Figure 7.
(A) Model for MSCRAMM secretion and incorporation into the cell wall. The domain organization of a typical MSCRAMM is shown at the bottom. MSCRAMMs have an N-terminal Sec signal sequence for translocation across the cytoplasmic membrane. The protein remains anchored in the cytoplasmic membrane by the CWSS. The positively charged C terminus remains in the cytoplasm, orienting the LPXTG motif to the extracellular side of the membrane. The SrtA sortase cleaves between the Thr and Gly of the MSCRAMM LPXTG motif, forming a covalent thioacyl intermediate. The MSCRAMM is then transferred to a lipid II peptidoglycan precursor and finally integrated into the cell wall at an amino acid cross-bridge. (B) Crystal structures of the Ace and UafA MSCRAMMs (PDB IDs: 2Z1P and 3IRP, respectively). The upper structure shows the N1 and N2 subdomains of Ace in blue and green, respectively; the yellow circle represents bound collagen. Both domains have DEv-Ig folds. The C terminus of the N2 subdomain inserts into the N1 subdomain, forming a latch. The lower structure depicts the N2, N3 and B subdomains of UafA. The N2 and N3 subdomains adopt DEv-Ig folds and the B subdomain adopts a variant of the IgG-rev fold. The loop connecting the N3 and B domains (cyan) is thought to insert into the N2 subdomain upon ligand binding to form a latch.