Figure 1.
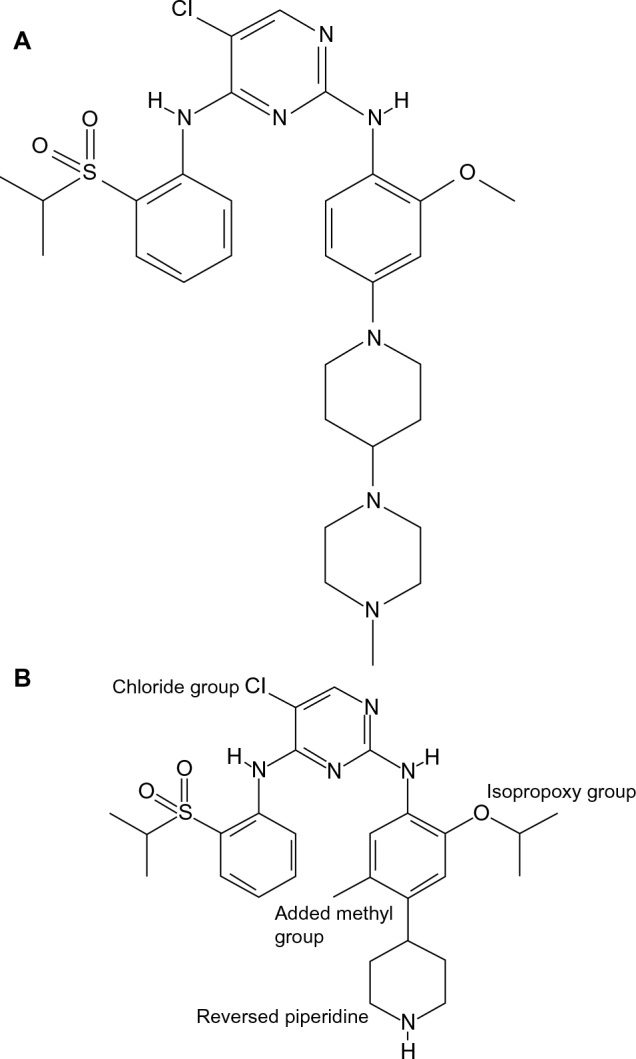
Chemical structure of TAE684 (A) and LDK378 (B).
Notes: This figure depicts the structural determinants of Ceritinib potency. The group isopropoxy at the aniline ring confers an improved kinase selectivity of Ceritinib, whereas the reversal of piperidine along with the methyl group para the isopropoxy are thought to minimize the possibility of reactive adducts formation.
