Abstract
PURPOSE
Potentially inappropriate prescribing (PIP) is common in older people and can result in increased morbidity, adverse drug events, and hospitalizations. The OPTI-SCRIPT study (Optimizing Prescribing for Older People in Primary Care, a cluster-randomized controlled trial) tested the effectiveness of a multifaceted intervention for reducing PIP in primary care.
METHODS
We conducted a cluster-randomized controlled trial among 21 general practitioner practices and 196 patients with PIP. Intervention participants received a complex, multifaceted intervention incorporating academic detailing; review of medicines with web-based pharmaceutical treatment algorithms that provide recommended alternative-treatment options; and tailored patient information leaflets. Control practices delivered usual care and received simple, patient-level PIP feedback. Primary outcomes were the proportion of patients with PIP and the mean number of potentially inappropriate prescriptions. We performed intention-to-treat analysis using random-effects regression.
RESULTS
All 21 practices and 190 patients were followed. At intervention completion, patients in the intervention group had significantly lower odds of having PIP than patients in the control group (adjusted odds ratio = 0.32; 95% CI, 0.15–0.70; P = .02). The mean number of PIP drugs in the intervention group was 0.70, compared with 1.18 in the control group (P = .02). The intervention group was almost one-third less likely than the control group to have PIP drugs at intervention completion, but this difference was not significant (incidence rate ratio = 0.71; 95% CI, 0.50–1.02; P = .49). The intervention was effective in reducing proton pump inhibitor prescribing (adjusted odds ratio = 0.30; 95% CI, 0.14–0.68; P = .04).
CONCLUSIONS
The OPTI-SCRIPT intervention incorporating academic detailing with a pharmacist, and a review of medicines with web-based pharmaceutical treatment algorithms, was effective in reducing PIP, particularly in modifying prescribing of proton pump inhibitors, the most commonly occurring PIP drugs nationally.
Keywords: randomized controlled trial, potentially inappropriate prescribing, primary health care, prescription drugs, practice-based research
INTRODUCTION
Older people tend to have multimorbidity with consequent polypharmacy, making prescribing in this population challenging, with the potential for adverse outcomes including drug-drug interactions and adverse drug events (ADEs).1,2 Potentially inappropriate prescribing (PIP) describes a number of suboptimal prescribing practices, particularly the use of medicines that introduce a greater risk of ADEs when a safer, as effective alternative is available to treat the same condition.3,4 PIP in older people is common across health care settings and can result in increased morbidity, ADEs, and hospitalizations.2,5,6 In Ireland, 36% of those aged 70 years or older received at least 1 potentially inappropriate prescription in 2007, with an associated expenditure of more than €45 million.7 PIP in community-dwelling older Irish people is associated with increased ADEs and accident and emergency visits, and poorer health-related quality of life.8
Interventions targeting PIP represent an important public health measure, particularly in primary care, where the majority of prescribing takes place. No single interventional strategy has proved to be most effective.9 A number of commentators have argued that a multifaceted intervention, which combines a number of techniques within a single intervention,10 may be more likely to improve prescribing than any one intervention alone.11,12 To date, a limited number of multifaceted interventions have been evaluated in primary care to decrease PIP.13,14
The purpose of the OPTI-SCRIPT study (Optimizing Prescribing for Older People in Primary Care, a cluster-randomized controlled trial) was to investigate the effectiveness of a multifaceted intervention in reducing PIP in older people in Irish primary care.
METHODS
We conducted a cluster-randomized controlled trial in Irish primary care to alter general practitioner (GP) PIP-related prescribing following the Consolidated Standards of Reporting Trials (CONSORT) guidelines.15 The study protocol and intervention development have been detailed previously.16,17 The Research Ethics Committee of the Irish College of General Practitioners approved the study.
Recruitment and Randomization
GP practices from the Health Research Board Centre for Primary Care Research network were invited to participate by e-mail with a follow-up telephone call. Practices were eligible if they had at least 80 patients aged 70 years or older and were based in greater Dublin. Consenting practices were instructed to randomly select 50 patients from this age-group with capacity to provide informed consent. Prescriptions of these patients were assigned a study ID and sent to the research team, where the research pharmacist determined if they had PIP (Supplemental Appendix 1, available at http://www.annfammed.org/content/13/6/545/suppl/DC1).16 Eligible patients were sent study information packs by the practice, and those wishing to participate returned signed consent forms to the research team.
Baseline data were collected before allocation. Practices were allocated to intervention and control groups by an independent researcher using minimization,18 an allocation method commonly used in cluster-randomized controlled trials to ensure balanced allocation of important cluster-level attributes such as practice size when cluster numbers are small. It was not possible to blind patients or GPs to allocations; however, the outcome assessor was blinded.
Intervention and Control Groups
The multifaceted intervention involved academic detailing with a pharmacist on how GPs can review medicines with participating patients; the medicine reviews were supported by web-based pharmaceutical treatment algorithms for GPs that provided evidence-based alternative treatment options to PIP drugs, and tailored patient information leaflets (Table 1 and Supplemental Appendix 2).16 The intervention was delivered from October 2012 to September 2013. Control practices delivered usual care and received one-time simple patient-level PIP feedback (Table 1).
Table 1.
Summary of OPTI-SCRIPT Intervention and Control Procedures
| Intervention | Control |
| Academic detailing with a pharmacist, which entailed one 30-min session in which a pharmacist visited the practice to discuss PIP, a review of medicines, and the web-based pharmaceutical treatment algorithms Medicine review with web-based pharmaceutical treatment algorithms. GPs were asked to conduct 1 review per patient using the web-based platform to guide them through the process. The GP was presented with the specific PIP drug(s) for each patient, and for each PIP drug, was offered a treatment algorithm with the following structure: 1. The individual PIP with reason for concern 2. Alternative pharmacologic and nonpharmacologic treatment options 3. Background information (where relevant) Patient information leaflets to give to patients during the review. Each leaflet: 1. Described the PIP and the reasons why it may be inappropriate 2. Outlined the alternative pharmacologic and nonpharmacologic therapies GPs may offer |
Delivery of usual care, which for public general medical services patients allows GPs to give a prescription on a monthly or 3-month basis Receipt of simple, patient-level PIP postal feedback in the form of a list summarizing the medication class to which the individual patient’s potentially inappropriate medication belonged No academic detailing visit, and no prompts to carry out a medicine review with the individual patients |
GP = general practitioner; OPTI-SCRIPT = Optimizing Prescribing for Older People in Primary Care, a cluster-randomized controlled trial; PIP = potentially inappropriate prescribing.
Outcomes
Outcome data were collected at intervention completion (ie, the time point at which all reviews were completed in a practice), which occurred approximately 4 to 6 months after baseline data collection.
Two primary outcomes were used. First, we assessed the proportion of patients with PIP drugs, a composite measure that captured any number of PIP drugs as included in the study to address multiple PIP in individual patients. Second, we assessed the mean number of PIP drugs per group. PIP was determined for intervention and control groups from a review of prescriptions by a research pharmacist (Supplemental Appendix 1).
Secondary outcomes included individual measures of the composite drug-specific outcome, including the absolute number of PIP drugs per group of the top 5 reported nationally7: proton pump inhibitor at full therapeutic dosage for more than 8 weeks, long-term use (>3 months) of nonsteroidal anti-inflammatories (NSAIDs), long-term use (>1 month) of long-acting benzodiazepines, therapeutic duplication, and tricyclic antidepressants with an opiate or calcium channel blocker. Patient-reported outcomes included responses on the Patients’ Beliefs about Medicines Questionnaire19 and the Well-Being Questionnaire20 collected via self-completed questionnaires.
Sample Size Calculation
We needed a sample of at least 22 practices and 220 patients, incorporating the effects of cluster randomization and a 10% loss to follow-up. The calculation was based on both primary outcomes. The calculation for the proportion of participants with PIP was based on demonstrating a clinically relevant 10% absolute reduction (from 100% to 90%) in the proportion of PIP, with 80% power and a statistical significance of 5% (1-sided), between randomized groups. For the mean, the calculation was based on demonstrating a 30% relative reduction in the mean number of potentially inappropriate prescriptions in the intervention group compared with the control group (equivalent to a mean reduction of 1.02 inappropriate prescriptions), with 80% power and a statistical significance of 5% (2-sided).17
Analyses
We performed analyses according to intention to treat. Separate approaches were used to analyze the 2 primary outcomes. The proportion of patients with PIP is presented and was analyzed using random-effects logistic regression with the individual as the unit of analysis and the practice included as the random effect, to control for the effects of clustering. We included in the model baseline covariates (age, sex, baseline number of PIP drugs, baseline number of repeat medications) and minimization factors (number of GPs, practice location).
The mean number of PIP drugs was calculated per group, as specified in the study protocol, and a mean difference was calculated using a cluster-level t test.17 Preliminary analyses, however, indicated that the data were skewed. The median number of PIP drugs was additionally investigated and skewness was addressed using random-effects Poisson regression, presenting incidence rate ratios. Again, the individual was the unit of analysis and the practice was included as the random effect, and baseline covariates and minimization factors were included. We used the Bonferroni correction to adjust for multiple comparisons.
Random-effects logistic regression analyses were used to test the differences in drug-specific secondary outcomes between intervention and control groups, and random-effects multiple regression analyses were conducted for the patient-reported outcomes.
National Contemporaneous Comparison Group
The control group may have changed their prescribing behavior because of the reactive effects of being studied (the Hawthorne effect) and receiving simple feedback.21–23 In anticipation of this possibility, we analyzed anonymized data from the Primary Care Reimbursement Service (PCRS) pharmacy claims database of dispensed medications (a national prescribing database of GP and pharmacy claims),24 as a national contemporaneous comparison group. National PCRS prescribing data for those aged 70 years and older from September 2012 to August 2013 were analyzed, and the following data were retrieved: number of people with PIP, number of people with no PIP, decreases in the number of PIP drugs, and PIP that remained the same or increased.
PIP was assessed using 28 criteria from the OPTI-SCRIPT study16 (6 of the criteria could not be applied as the PCRS data lacked the detailed information needed). From these values, we calculated crude odds ratios (ORs), comparing the OPTI-SCRIPT intervention group with the national PCRS comparator.
RESULTS
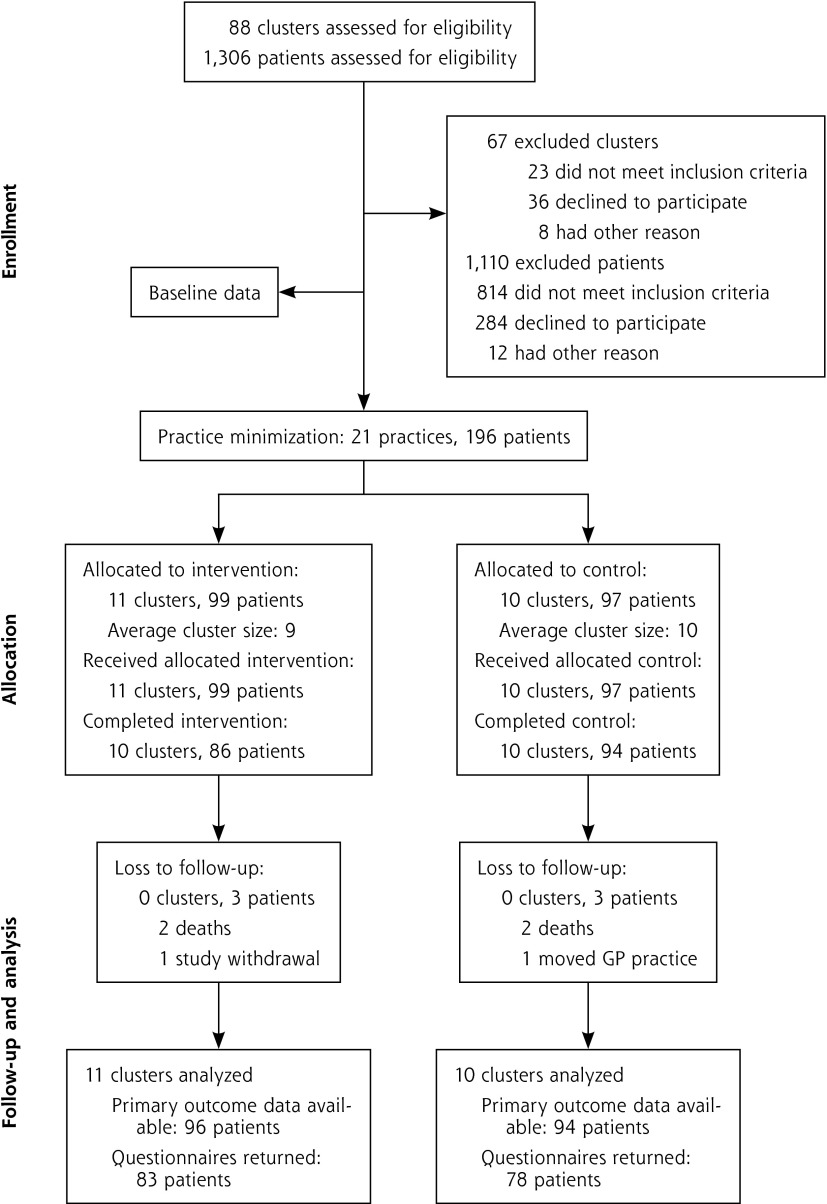
Figure 1 shows the flow of participants through our trial. In total, 21 GP practices and 196 patients were recruited. All practices and 190 patients (97%) had follow-up through intervention completion. Practices and patients were similar at baseline, but the control practices were situated in more socioeconomically deprived areas (Table 2). Receipt of proton pump inhibitors at maximum therapeutic dosage for more than 8 weeks was the most frequently occurring PIP in both groups (Table 3).
Figure 1.
Flow of practices and patients through the study.
GP = general practitioner.
Table 2.
Baseline Characteristics of Practices and Patients in Intervention and Control Groups
| Characteristic | Intervention Group | Control Group |
|---|---|---|
| Practices | n = 11 | n = 10 |
| GMS list size, No. (%) | ||
| ≤500 | 1 (9.1) | 2 (20.0) |
| 501–1,500 | 3 (27.3) | 2 (20.0) |
| ≥1,501 | 7 (63.6) | 6 (60.0) |
| Have a manager, No. (%) | 8 (72.8) | 7 (70.0) |
| Location, No. (%) | ||
| Urbana | 8 (80.0) | 8 (72.7) |
| Mixed | 3 (20.0) | 2 (27.3) |
| GPs per practice, mean (SD) | 4.1 (3.1) | 4.1 (2.1) |
| Patients >70 years old per practice, mean (SD) | 712.1 (525.3) | 788.2 (987.2) |
| Deprivation score, median (IQR)b | 0.5 (–0.3 to 1.6) | 1.4 (0.3 to 2.4) |
| Patients | n = 99 | n = 97 |
| Male, No. (%) | 55 (55.6) | 50 (51.5) |
| Marital status, No. (%) | ||
| Married | 56 (56.6) | 51 (53.1) |
| Widowed | 26 (26.3) | 32 (33.3) |
| Single | 14 (14.1) | 10 (10.4) |
| GMS card holder, No. (%) | 88 (88.9) | 95 (97.9) |
| Age, mean (SD) | 77.1 (4.9) | 76.4 (4.8) |
| Repeat medications, mean (SD) | 10.2 (4.5) | 9.5 (4.1) |
| PIP drugsc | ||
| Mean (SD) | 1.31 (0.6) | 1.39 (0.6) |
| Median (IQR) | 1 (1–2) | 1 (1–2) |
| Most prevalent PIP drug: proton pump inhibitors, No. (%) | 53 (53.3) | 65 (67.7) |
GMS = general medical services; GP = general practitioner; IQR = interquartile range; PIP = potentially inappropriate prescribing.
Note: Figures are numbers (percentages) unless stated otherwise.
Urban area: relatively small center of population, with at least 5,000 residents.37
Population-weighted deprivation score for each practice; higher scores mean practices are situated in more socioeconomically deprived areas.
All patients had at least 1 potentially inappropriate prescription at baseline.
Table 3.
Potentially Inappropriate Prescriptions at Baseline in Intervention and Control Groups
| Potentially Inappropriate Prescription | Intervention Group, No. (%) (n = 99) | Control Group, No. (%) (n = 97) |
|---|---|---|
| Proton pump inhibitor at maximum therapeutic dosage for >8 weeks | 53 (53.3) | 65 (67.7) |
| NSAIDs: long-term use, interactions with certain medications (eg, diuretic) | 21 (21.2) | 16 (16.8) |
| Therapeutic duplication: any regular duplicate drug class prescription (eg, 2 concurrent opiates, NSAIDs) | 19 (19.2) | 13 (13.5) |
| Long-term use (>1 month) of long-acting benzodiazepines | 14 (14.4) | 8 (8.3) |
| Steroid without bisphosphonate | 9 (9.1) | 4 (4.2) |
| Bladder antimuscarinics: contraindications and interactions with certain medications | 1 (1.0) | 9 (9.4) |
| Prolonged use (>1 week) of first-generation antihistamines | 4 (4.0) | 2 (2.1) |
| Tricyclic antidepressants: contraindications and interactions with certain medications (eg, opiate, calcium channel blocker) | 1 (1.0) | 5 (5.2) |
| Thiazide diuretic in patient with gout | 3 (3.0) | 2 (2.1) |
| Aspirin: contraindications and interactions with certain medications (eg, warfarin) | 3 (3.0) | 1 (1.0) |
| Digoxin: inappropriate dose | 1 (1.0) | 3 (3.1) |
| Calcium channel blocker: contraindications and interactions with certain medications | 0 (0.0) | 3 (3.1) |
NSAID = nonsteroidal anti-inflammatory drug.
Primary Outcomes
At intervention completion, the percentage of patients having PIP was 52% in the intervention group compared with 77% in the control group (Table 4). Intervention patients had significantly lower odds of having PIP than control counterparts (adjusted OR = 0.32; 95% CI, 0.15–0.70; P = .02).
Table 4.
Intention-to-Treat Analysis of Primary Outcomes
| Outcome | Intervention Group, No. (%) | Control Group, No. (%) | Adjusteda Odds Ratio (95% CI) | Adjusteda Incidence Rate Ratio (95% CI) | Mean Differencea,b (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Primary outcome: proportion with PIP | ||||||
| Baseline | 99 (100) | 97 (100) | – | – | – | – |
| Intervention completion | 52 (52.5) | 75 (77.3) | 0.32 (0.15–0.70) | – | – | .02 |
| Primary outcome: mean (SD) PIP | ||||||
| Baseline | 1.31 (0.6) | 1.39 (0.6) | – | – | – | – |
| Intervention completion | 0.70 (0.1) | 1.18 (0.1) | – | – | −0.48 (–0.80 to –0.17) | .02 |
| Additional outcome: median (IQR) PIP per patient | ||||||
| Baseline | 1 (1–2) | 1 (1–2) | – | – | – | – |
| Intervention completion | 1 (0–1) | 1 (1–2) | – | – | – | – |
| Additional outcome: Poisson regression | ||||||
| Baseline | 99 (100) | 97 (100) | – | – | – | – |
| Intervention completion | 52 (52.5) | 75 (77.3) | – | 0.71 (0.50–1.02)c | – | .49 |
IQR = interquartile range; PIP = potentially inappropriate prescribing.
Note: Values are numbers (percentages) unless stated otherwise. We used the Bonferroni method to account for multiple comparisons.
Adjusted for age, sex, baseline number of PIP drugs, baseline number of repeat medications, number of general practitioners, and practice location.
Results from modeling the number of PIP drugs per patient with Poisson regression analysis adjusted for age, sex, baseline number of PIP drugs, baseline number of repeat medications, number of general practitioners, and practice location.
Results from unadjusted cluster-level t test.
The mean number of PIP drugs in the intervention group was 0.70, compared with 1.18 in the control group (P = .02). The median was 1 in both groups. When we applied Poisson regression analysis, the estimated number of PIP drugs was 29% lower in the intervention group than in the control group, but this difference was not significant (incidence rate ratio = 0.71; 95% CI, 0.50–1.02; P = .49) (Table 4).
Secondary Outcomes
At intervention completion, patients in the intervention group had significantly lower odds of receiving potentially inappropriate proton pump inhibitors compared with those in the control group (adjusted OR = 0.30; 95% CI, 0.14–0.68, P = .04) (Table 5). No significant differences were found for other drug-specific outcomes. In the intervention group, the potentially inappropriate proton pump inhibitors were amended by dose reduction to a maintenance level in 50% of patients, were stopped completely in 20%, were switched to an alternative (eg, histamine 2 antagonist) in 11%, and were unaltered in 20%.
Table 5.
Intention-to-Treat Analysis of Secondary Outcomes
| Outcome | Intervention Group | Control Group | Adjusteda Odds Ratio or Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| Drug-specific outcomes | ||||
| Proton pump inhibitor, No. (%) | ||||
| Baseline | 53 (53.5) | 65 (67.7) | ||
| Intervention completion | 23 (23.2) | 46 (47.4) | 0.30 (0.14–0.68) | .04 |
| Duplicate, No. (%) | ||||
| Baseline | 19 (19.2) | 13 (13.5) | ||
| Intervention completion | 11 (11.1) | 11 (11.3) | 0.83 (0.32–2.13) | .99 |
| Long-term benzodiazepines, | ||||
| No. (%) | ||||
| Baseline | 14 (14.1) | 8 (8.1) | ||
| Intervention completion | 9 (9.1) | 9 (9.1) | 1.31 (0.47–3.68) | .99 |
| Patient-reported outcomes | ||||
| WBQ-12 score: mean well-beingb | ||||
| Baseline | 24.3 | 24.4 | ||
| Intervention completion | 23.6 | 24.0 | –0.41 (−0.80 to 1.07) | .99 |
| BMQ score: median necessity-concern differentialc | ||||
| Baseline | 7.0 | 5.8 | ||
| Intervention completion | 6.0 | 6.0 | 0.16 (−1.85 to 1.07) | .99 |
BMQ = Beliefs About Medicine Questionnaire; WBQ-12 = 12-item Well-Being Questionnaire.
Note: Figures are numbers (percentages) unless stated otherwise. The Bonferroni method was used to account for multiple comparisons.
Adjusted for age, sex, baseline number of PIP drugs, baseline number of repeat medications, number of general practitioners, and practice location.
Well-being score ranges from 0 to 36 (1–12 low, 13–24 medium, 25–36 high).
Scale from −20 to 20, where positive scores indicate benefits outweigh risks.
For the patient-reported outcomes of well-being and beliefs about medication, we found no significant differences between groups at completion of the intervention (Table 5).
National Contemporaneous Comparison Group
Participants in the OPTI-SCRIPT intervention group had lower odds of having PIP compared with those in the national comparator group (crude OR = 0.4; 95% CI, 0.3–0.6) and were more likely to have a decrease in the number of PIP drugs than the national comparator group (crude OR = 2.5; 95% CI, 1.8–4.0) (Table 6).
Table 6.
Comparison of PIP in the OPTI-SCRIPT Study Population With the PCRS National Comparator
| PIP Outcome | OPTI-SCRIPT Intervention Group | OPTI-SCRIPT Control Group | PCRS National Comparator |
|---|---|---|---|
| Presence of PIP | |||
| PIP at baseline | 99 (100) | 97 (100) | 103,261 (100) |
| PIP at intervention completion | 52 (52.5) | 75 (77.3) | 75,401 (73.1) |
| No PIP at intervention completion | 47 (47.5) | 22 (22.7) | 27,860 (26.9) |
| Crude odds ratio (95% CI) | 0.4 (0.3–0.6) | – | 1.0 (ref) |
| Decrease in PIP | |||
| PIP at baseline | 99 (100) | 97 (100) | 103,261 (100) |
| PIP same or increased at intervention completion | 42 (42.4) | 65 (67.0) | 67,188 (65.1) |
| PIP decreased at intervention completion | 57 (57.6) | 32 (32.9) | 36,073 (34.9) |
| Crude odds ratio (95% CI) | 2.5 (1.8–4.0) | – | 1.0 (ref) |
OPTI-SCRIPT = Optimizing Prescribing for Older People in Primary Care, a cluster-randomized controlled trial; PCRS = Primary Care Reimbursement Services; PIP = potentially inappropriate prescribing; ref = reference group.
Note: Numbers (percentages) of participants are presented, unless otherwise stated.
DISCUSSION
Main Findings
The OPTI-SCRIPT intervention was effective in reducing PIP. This effect, however, was mediated principally through reducing prescriptions of proton pump inhibitors used at maximal dose, the most commonly encountered PIP in this study.
Previous studies aimed at reducing PIP have focused on hospital and nursing home settings.9,25,26 A limited number of randomized controlled trials to reduce PIP specifically in primary care have been conducted. Of those interventions that have been evaluated, single interventions such as computerized decision-support systems, educational interventions, and multidisciplinary teams have produced inconsistent effects.2,27,28 Multifaceted interventions may be more likely to improve prescribing than single interventions.11,12
Our results are consistent with 2 randomized controlled trials published since the start of the OPTI-SCRIPT study in finding a multifaceted intervention to be effective.13,14 In the Rx-PAD study, Rognstad et al14 and Straand et al29 found peer academic detailing, delivered at continuing medical education meetings, with mailed prescriber feedback, produced a 10% (95% CI, 5.9%–15.0%) reduction in PIP. Bregnhøj et al13 found interactive educational meetings and feedback resulted in a 5-point (95% CI, 7.3- to 2.6-point) improvement in the medication appropriateness index score. Differences in effect sizes reported between these studies and the OPTI-SCRIPT findings may arise from a number of factors, including differences in the criteria used to assess PIP, the duration of follow-up, and the included patients.
Another important difference may be the intensity of the intervention. OPTI-SCRIPT was more intensive, delivering academic detail face-to-face, rather than in a group setting. During the medication reviews, GPs were provided directly with patient-specific lists of PIP drugs and advice on medication changes via the web-based pharmaceutical treatment algorithms; provision of these resources may have yielded a larger effect size as it encouraged immediate action rather than simply providing educational support or information.
Changes in the prescribing of particular drugs can be responsible for the overall effectiveness of interventions.30 The OPTI-SCRIPT intervention primarily affected proton pump inhibitor PIP, which was highly prevalent at baseline (60%). We found no impact on therapeutic duplication or benzodiazepine use, likely because of the small numbers of patients exposed to these PIP drugs. Lack of change may also reflect, however, the different challenges of modifying medicines as opposed to altering dosage regimes, particularly with benzodiazepines, which have tolerance levels that pose challenges to interventions designed to improve prescribing.31 There is a concern that discontinuation of benzodiazepines in this population may produce more harm than benefit, and patients may be reluctant to stop the medicine.32,33 OPTI-SCRIPT GPs may have been more comfortable altering proton pump inhibitors than benzodiazepines. On the basis of the low number of benzodiazepines in this study, we cannot be certain that our intervention would be effective in reducing prescribing of these medications.
The OPTI-SCRIPT intervention did not have any measurable impact on patients’ sense of well-being and beliefs about medicine. The sample size may have been too small and the follow-up period too short to detect a difference in patient-reported outcomes, a common criticism of prescribing interventions.27 It is possible that patients’ beliefs about medicines may have been more likely to change than patients’ well-being, given the short follow-up period; however, evidence indicates that beliefs about medicines remain stable over time, irrespective of changes in health status.34 Overall, these results suggest that modifications in proton pump inhibitor dosage do not appear to affect patients’ sense of well-being or concerns.
Strengths and Limitations
Strengths of this study include a rigorous design of a clinically relevant intervention,16 high retention rates (primarily due to the nature of the outcome data and the short follow-up period), completeness of the prescription data, and conduct in a primary care setting using existing resources. Selection bias was minimized by collecting baseline data before minimization, which was carried out by an independent third party. Owing to the nature of the intervention, it was not possible to blind patients or GPs to allocation; however, the outcome assessor was blinded to allocation.
The intervention was effective at decreasing the most prevalent PIP in this study. The more frequent an outcome is, the greater the potential the OR will overestimate or underestimate the relative risk. Using methods proposed by Zhang and Yu,35 we explored this possibility and found little difference between the OR (0.32) and the relative risk (0.38), increasing confidence in our study findings.
Although the analysis of an outside comparison group (PCRS patients) provided a national context to the study, the findings revealed no notable difference overall in prescribing behavior by the intervention group compared with prescribing nationally. As this was a nonrandomized group, its composition may be subject to confounding.
The external validity of this study may have limitations. In all, 32% of invited GP practices were recruited; this value compares favorably with that of similar PIP-related randomized controlled trials13 but is lower than that reported in other primary care studies.36 When compared with a national sample of practices,37 study practices had, on average, more GPs and public patients, so they may not be representative of practices nationally. The last available national data on GPs, however, was from 2005, and may therefore be somewhat out of date.37
Implications for Practice and Directions for Future Study
The reduction in PIP in the OPTI-SCRIPT study may have important clinical and economic implications. Almost one-half of the intervention group were no longer exposed to PIP at intervention completion. Although we cannot assume that a change in PIP alters health outcomes,38,39 reducing PIP potentially may decrease adverse outcomes such as ADEs and hospitalizations in older patients.5 As the OPTI-SCRIPT study effect size was largely driven by proton pump inhibitor prescribing, the intervention may attenuate the risks associated with these drugs such as hip fractures and community-acquired pneumonia.40,41 Reducing PIP-related to proton pump inhibitors may also contribute to substantial savings as an estimated €22 million was spent on potentially inappropriate proton pump inhibitors in 2007.42
Given the positive findings presented here, we plan to further model the intervention components to determine the effectiveness of the OPTI-SCRIPT intervention long term, and the potential impact it may have on cases of PIP other than for proton pump inhibitor prescribing.
In conclusion, PIP is an important public health concern that can result in increased morbidity, ADEs, hospitalizations, and expenditure.2,7 This study shows that the OPTI-SCRIPT intervention reduced PIP, primarily by reducing proton pump inhibitor prescribing, in a way that is acceptable to both GPs and their patients. Tailoring of the intervention to influence more specifically different cases of PIP is planned.
Acknowledgments
David Williams, MD, Ronan McDonnell, PhD, and Daniel Clear, MB, contributed to the design of the intervention. Mary-Claire Kennedy, BSc (Pharm), conducted the academic detailing with GPs. Nicola Motterlini (deceased) provided statistical support at the study inception. David Williams is Consultant Stroke Physician, Geriatric Medicine – Beaumont hospital, Royal College of Surgeons in Ireland. Ronan McDonnell, Daniel Clear, and Mary-Claire Kennedy are all affiliated with the HRB Centre for Primary Care Research. We thank the HSE-PCRS for supplying the data for national comparison; in particular, we would also like to thank Frank Moriarty, Caitriona Cahir, and Kathleen Bennett. We thank all the GP practices and patients who participated in this study.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study is independent research, funded by the Health Research Board (HRB) PhD Scholars Programme in Health Services Research under grant PHD/2007/16 and the HRB Centre for Primary Care Research under grant HRC/2007/1.
Previous presentations: Clyne B, Smith SM, Hughes CM, et al. Effectiveness of a multifaceted intervention on potentially inappropriate prescribing in older patients in primary care: a cluster randomized controlled trial (the OPTI-SCRIPT study). Presented at: North American Primary Care Research Group (NAPCRG) Annual Conference; November 21–24, 2014; New York, New York; and at the Society for Academic Primary Care (SAPC) Annual Conference; July 9–11, 2014; Edinburgh, Scotland.
Clyne B, Cooper J, Hughes CM, Fahey TP, Smith SM. A process evaluation of a cluster randomized trial to reduce potentially inappropriate prescribing in older patients in primary care (OPTI-SCRIPT study). Presented at: Society for Academic Primary Care (SAPC) Annual Conference; July 9–11, 2014; Edinburgh, Scotland.
Clyne B, Smith SM, Hughes CM, Bradley MC, Boland F, Fahey TP. Addressing potentially inappropriate prescribing in primary care: results from the OPTI-SCRIPT cluster randomized controlled trial. Presented at: Irish Gerontological Society (IGS) Annual and Scientific Meeting; October 9–11, 2014; Galway, Ireland; and at the Association of Departments of General Practice in Ireland (AUDGPI) Annual Scientific Meeting; March 6–7, 2014; Cork, Ireland.
Clyne B, Bradley MC, Smith SM, Hughes CM, Fahey TP. Feasibility of medicines review to reduce potentially inappropriate medicines in the elderly: the OPTI-SCRIPT cluster randomized controlled trial. Presented at: International Society For Pharmacoeconomics and Outcomes Research (ISPOR) Annual European Congress; November 2–6, 2013; Dublin, Ireland.
Trial registration: Current controlled trials ISRCTN41694007.
Supplementary materials: Available at http://www.AnnFamMed.org/content/13/6/545/suppl/DC1/.
References
- 1.Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336(7644):606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–184. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann CP, Tremp R, Hersberger KE, Lampert ML. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol. 2014;70(1):1–11. [DOI] [PubMed] [Google Scholar]
- 4.O’Mahony D, Gallagher PF. Inappropriate prescribing in the older population: need for new criteria. Age Ageing. 2008;37(2):138–141. [DOI] [PubMed] [Google Scholar]
- 5.Jano E, Aparasu RR. Healthcare outcomes associated with Beers’ criteria: a systematic review. Ann Pharmacother. 2007;41(3):438–447. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse drug events in older people. BMC Geriatr. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahir C, Bennett K, Teljeur C, Fahey T. Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br J Clin Pharmacol. 2014;77(1):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcum ZA, Handler SM, Wright R, Hanlon JT. Interventions to improve suboptimal prescribing in nursing homes: A narrative review. Am J Geriatr Pharmacother. 2010;8(3):183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia RM. Five ways you can reduce inappropriate prescribing in the elderly: a systematic review. J Fam Pract. 2006;55(4):305–312. [PubMed] [Google Scholar]
- 11.Majumdar SR, Soumerai SB. Why most interventions to improve physician prescribing do not seem to work. CMAJ. 2003;169(1):30–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8):(8 Suppl 2):II2–45. [PubMed] [Google Scholar]
- 13.Bregnhøj L, Thirstrup S, Kristensen MB, Bjerrum L, Sonne J. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. Eur J Clin Pharmacol. 2009;65(2):199–207. [DOI] [PubMed] [Google Scholar]
- 14.Rognstad S, Brekke M, Fetveit A, Dalen I, Straand J. Prescription peer academic detailing to reduce inappropriate prescribing for older patients: a cluster randomised controlled trial. Br J Gen Pract. 2013;63(613):e554–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell MK, Elbourne DR, Altman DG; CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328(7441):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clyne B, Bradley MC, Hughes CM, et al. ; OPTI-SCRIPT study team. Addressing potentially inappropriate prescribing in older patients: development and pilot study of an intervention in primary care (the OPTI-SCRIPT study). BMC Health Serv Res. 2013;13(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clyne B, Bradley MC, Smith SM, et al. ; OPTI-SCRIPT Study Team. Effectiveness of medicines review with web-based pharmaceutical treatment algorithms in reducing potentially inappropriate prescribing in older people in primary care: a cluster randomized trial (OPTI-SCRIPT study protocol). Trials. 2013;14(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saghaei M. An overview of randomization and minimization programs for randomized clinical trials. J Med Signals Sens. 2011;1(1): 55–61. [PMC free article] [PubMed] [Google Scholar]
- 19.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. [Google Scholar]
- 20.Pouwer F, van der Ploeg HM, Adèr HJ, Heine RJ, Snoek FJ. The 12-item well-being questionnaire. An evaluation of its validity and reliability in Dutch people with diabetes. Diabetes Care. 1999;22(12):2004–2010. [DOI] [PubMed] [Google Scholar]
- 21.Soumerai SB, McLaughlin TJ, Avorn J. Improving drug prescribing in primary care: a critical analysis of the experimental literature. Milbank Q. 1989;67(2):268–317. [PubMed] [Google Scholar]
- 22.Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;(2):CD000259. [DOI] [PubMed] [Google Scholar]
- 23.Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Health Service Executive. Primary Care Reimbursement Service Statistical Analysis of Claims and Payments 2011. Dublin, Ireland: Health Service Executive; 2011. [Google Scholar]
- 25.Alldred DP, Raynor DK, Hughes C, Barber N, Chen TF, Spoor P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2013;2(2):CD009095. [DOI] [PubMed] [Google Scholar]
- 26.Forsetlund L, Eike MC, Gjerberg E, Vist GE. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatr. 2011;11(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yourman L, Concato J, Agostini JV. Use of computer decision support interventions to improve medication prescribing in older adults: a systematic review. Am J Geriatr Pharmacother. 2008;6(2):119–129. [DOI] [PubMed] [Google Scholar]
- 28.Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26(12):1013–1028. [DOI] [PubMed] [Google Scholar]
- 29.Straand J, Fetveit A, Rognstad S, Gjelstad S, Brekke M, Dalen I. A cluster-randomized educational intervention to reduce inappropriate prescription patterns for elderly patients in general practice—The Prescription Peer Academic Detailing (Rx-PAD) study [NCT00281450]. BMC Health Serv Res. 2006;6(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raebel MA, Charles J, Dugan J, et al. Randomized trial to improve prescribing safety in ambulatory elderly patients. J Am Geriatr Soc. 2007;55(7):977–985. [DOI] [PubMed] [Google Scholar]
- 31.Smith AJ, Tett SE. Improving the use of benzodiazepines—is it possible? A non-systematic review of interventions tried in the last 20 years. BMC Health Serv Res. 2010;10:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22(3):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iliffe S, Curran HV, Collins R, Yuen Kee SC, Fletcher S, Woods B. Attitudes to long-term use of benzodiazepine hypnotics by older people in general practice: findings from interviews with service users and providers. Aging Ment Health. 2004;8(3):242–248. [DOI] [PubMed] [Google Scholar]
- 34.Porteous T, Francis J, Bond C, Hannaford P. Temporal stability of beliefs about medicines: implications for optimising adherence. Patient Educ Couns. 2010;79(2):225–230. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. [DOI] [PubMed] [Google Scholar]
- 36.Jones R, Jones RO, McCowan C, Montgomery AA, Fahey T. The external validity of published randomized controlled trials in primary care. BMC Fam Pract. 2009;10(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Dowd T, O’Kelly M, O’Kelly F. Structure of General Practice in Ireland 1982–2005. Dublin, Ireland: Trinity College Dublin; 2006. [Google Scholar]
- 38.Patterson SM, Hughes C, Kerse N, Cardwell C, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Sys Rev. 2012;5:CD008165. [DOI] [PubMed] [Google Scholar]
- 39.Scott IA, Gray LC, Martin JH, Pillans PI, Mitchell CA. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18(4):121–124. [DOI] [PubMed] [Google Scholar]
- 40.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24): 2947–2953. [DOI] [PubMed] [Google Scholar]
- 41.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955–1960. [DOI] [PubMed] [Google Scholar]
- 42.Cahir C, Fahey T, Tilson L, Teljeur C, Bennett K. Proton pump inhibitors: potential cost reductions by applying prescribing guidelines. BMC Health Serv Res. 2012;12(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]