Abstract
Descending noradrenergic inhibition to the spinal cord from the locus coeruleus (LC) is an important endogenous pain-relief mechanism which can be activated by local glutamate signaling. Here we tested whether dysregulation of extracellular glutamate level in the LC induced by down-regulating astroglial glutamate transporter-1(GLT-1) impairs endogenous analgesia. In rats treated with repeated LC injections of GLT-1 selective or non-targeting small interfering RNA (siRNA), a subdermal injection of capsaicin was used to examine noxious stimulation–induced analgesia (NSIA), evoked LC glutamate and spinal noradrenaline release, and evoked LC neuronal activity. LC-injected GLT-1 siRNA reduced expression of GLT-1 in the LC (P=0.02), increased basal activity of LC neurons (P<0.01), and increased basal extracellular concentrations of LC glutamate (P<0.01) and spinal noradrenaline (P<0.01), but did not affect mechanical withdrawal thresholds in the hindpaw (P=0.83), compared to non-targeting siRNA. LC-injected GLT-1 siRNA impaired capsaicin-evoked release of LC glutamate and spinal noradrenaline, capsaicin-evoked LC neuronal activation, and NSIA. These results suggest that astroglial GLT-1 is essential to normal LC function and that increased extracellular glutamate by down-regulating GLT-1 impairs evoked LC activity and NSIA, essentially taking the LC “off-line”.
Keywords: Locus coeruleus, astrocytes, glutamate transporter-1, endogenous analgesia, small interfering RNA
1. Introduction
As an important endogenous analgesic neurotransmitter, noradrenaline is released in the spinal cord from bulbospinal axons which mainly originate from the locus coeruleus (LC) in the brainstem, and stimulates α2 adrenoceptors to suppress spinal pain transmission [11]. Although endogenous analgesia is mediated by several peripheral and central mechanisms [4, 5, 21], we recently demonstrated in rats that activation of LC-spinal descending noradrenergic pathway is essential to noxious stimulation-induced analgesia (NSIA) [8, 16], which is induced by a subdermal injection of capsaicin and validated as a measure of endogenous analgesia in animals and humans [4, 9].
Among various neurochemical inputs modulating neuronal activity of noradrenergic neurons in the LC, glutamate is considered as a primary excitatory regulator, acting through AMPA receptors [19]. Glutamate also inhibits its own release from the glutamatergic terminal via group 2 and 3 metabotropic glutamate receptors (mGluRs) which are coupled to inhibitory G-proteins that inhibit adenylate cyclase [13]. Extracellular glutamate is classically removed by two types of astroglial glutamate transporters, primarily glutamate transporter-1 (GLT-1), but also glutamate-aspartate transporter [17, 18]. Although GLT-1 may express in glutamatergic terminals [10], astroglial GLT-1 provides the majority of functional glutamate transport and is essential for maintaining low extracellular glutamate in the central nervous system [18]. As such, knock-down or blockade of GLT-1 increases basal extracellular glutamate concentrations in the LC of normal rats [8, 20]. Similar to the results from exogenous manipulations of GLT-1, we recently demonstrated in rats with chronic neuropathic hypersensitivity that peripheral nerve injury decreases expression of GLT-1 and increases basal extracellular glutamate concentrations [8]. This increased basal glutamate level after nerve injury reduces noxious stimulation-evoked glutamate release via activation of presynaptic mGluRs, subsequently reducing evoked LC neuronal activity and spinal noradrenaline release to impair NSIA [8]. These results were consistent with clinical observations that patients with established neuropathic pain have a reduced ability to physiologically recruit descending inhibition [9] and suggest that the impaired responsivity of the LC to noxious stimuli in chronic neuropathic pain may be a consequence of down-regulation of GLT-1 in the LC after nerve injury. However, whether down-regulation of GLT-1 in the LC alone is sufficient to impair the LC responsivity to noxious stimulus in the absence of nerve injury or neuropathic pain has not been tested.
The current study examined whether the knock-down of GLT-1 expression in the LC of normal rats by selective small interfering RNA (siRNA) impairs NSIA and noxious stimuli-evoked release of glutamate in the LC, subsequent LC neuronal activation, and spinal noradrenaline release.
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN), weighing 220 to 280 g, were housed under a 12-hour light-dark cycle, with free access to food and water, and all studies were performed during the light portion of the cycle. All experiments were approved by the Animal Care and Use Committee at Wake Forest University (Winston-Salem, NC).
2.2 LC cannula implantation and siRNA treatments
LC cannula implantation and repeated LC injections of siRNA were performed as previously described [6, 20]. Briefly, under 2% isoflurane, a sterile stainless-steel guide cannula (CXG-8 for microdialysis, Eicom Co., Kyoto, Japan, or C315G for other experiments, Plastic one, Roanoke, VA) was implanted into the right LC (9.8mm posterior and 1.4mm lateral to the bregma, and 6.5 mm ventral from the surface of the dura mater), according to the rat brain atlas [15]. One week after the cannula implantation, a mixture of siRNAs for rat GLT-1 (8.3 pmol/ 0.5 μl, SMARTpool #M-091209-02, Thermo Fisher Scientific Inc., Pittsburgh, PA) or a non-targeting siRNA pool (8.3 pmol/ 0.5 μl, #D-001206-14, Thermo Fisher Scientific Inc.) was injected through the LC guide cannula for 5 consecutive days. Animals were randomized to treatment within cohorts. After the experiment, all animals received an intra-LC injection of methylene blue (0.5 µl) and were euthanized by an intravenous or intraperitoneal injection of pentobarbital (150 mg/kg). The brain was removed and sectioned, and the placement of the cannula was verified visually. This study included data from only animals (65 of 68 rats) with successful LC cannula placement.
2.3 Behavior test: NSIA
Rats were habituated over several days to testing to determine withdrawal threshold from the hindpaw using a Randall-Selitto analgesimeter (Ugo Basile, Comerio, Italy). NSIA was performed as previously reported [8]. Under brief isoflurane anesthesia (2%), capsaicin (150 μg/50 μl/rat; Sigma-Aldrich, St. Louis, MO) was injected into right forepaw, and the withdrawal thresholds in the right hindpaw were measured with prior to and 30 min after injection. The investigators measuring withdrawal thresholds were blinded to treatment.
2.4 Western blotting for GLT-1 in the LC
Western blotting for GLT-1 in the LC was performed as previously described [20]. Samples (25 μg protein) from the right LC and adjacent tissue were placed on 10% gels (Criterion Tris-HCl Gel; Bio-Rad, Hercules, CA), run at 100 V for 1 h, and transferred to polyvinylidene difluoride membrane (Bio-Rad). The membrane was incubated with a guinea pig anti-GLT-1 (1:5000, AB1783; Millipore, Billerica, MA) or a rabbit α-tubulin (1:5000, 2125S; Cell Signaling, Danvers, MA), followed by a corresponding horseradish peroxidase-conjugated secondary antibody (1:5000; anti-guinea pig or 1:5000; anti-rabbit, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Then the membrane was treated for 1 min with West Pico hemiluminescence substrate (Thermo Fisher Scientific Inc.), and exposed to X-ray film (Kodak BioMax film, Sigma-Aldrich). The density of each specific band was measured using a computer-assisted imaging analysis system (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA).
2.5 Microdialysis for LC glutamate and spinal noradrenaline
Simultaneous microdialysis in the spinal cord and LC in anesthetized rats and measurements for noradrenaline and glutamate in microdialysates was performed as previously described [8]. Under isoflurane anesthesia (2% for induction and 1.5 % for maintenance), the right L3-L6 level of spinal cord was exposed by the T13-L1 laminectomy. Microdialysis probes (CX-I-8-01, outer diameter = 0.22 mm, inner diameter = 0.20 mm, length = 1 mm; EICOM Co.) were inserted into the right spinal dorsal horn and right LC 1 h prior to the experiment and perfused with Ringer’s solution (1.0 μl/min). After two 30-min baseline samples, capsaicin, at the same dose used for NSIA, was injected into the right forepaw and samples obtained at 30 min intervals for 90 min. Glutamate and noradrenaline contents in the microdialysates were measured by separate high-pressure liquid chromatography systems with electrochemical detection (HTEC-500, EICOM Co.).
2.6 Immunohistochemistry
Capsaicin-evoked neuronal activation in the LC was examined according as previously described [8]. Under brief isoflurane anesthesia (2%), capsaicin, at the same dose used for NSIA, or vehicle (50 μl/rat; 50 % ethanol in saline) was injected into the right forepaw. Thirty minutes later, animals were euthanized with an intraperitoneal injection of pentobarbital (150 mg/kg) and perfused with 0.01 M phosphate-buffered saline containing 1% sodium nitrite, followed by 4% formalin in 0.1 M phosphate-buffered saline. The brainstems were dissected out, postfixed, cryoprotected, and sectioned at a 16 μm thickness. The sections were incubated with a rabbit monoclonal anti–phosphorylated cyclic adenosine monophosphate response element binding protein (pCREB) antibody (1:500, 06-519; Millipore, Billerica, MA) and a mouse monoclonal anti-dopamine-β-hydroxylase (DβH) antibody (1:500, MAB 308; Millipore), followed by Cy2 conjugated anti-mouse IgG (1:200, Jackson Immuno Research Laboratories) and Cy3 conjugated anti-rabbit IgG (1:600, Jackson Immuno Research Laboratories). Positive immunostaining for pCREB was defined using a constant threshold for each protein applied across all sections. DβH-immunoreactive (IR) cells with or without pCREB-IR were counted in the entire right LC from 4-5 sections per rat. The individual performing image quantification was blinded to treatment.
2.7 Statistical Analysis
Data are presented as mean ± SD. Microdialysis data were analyzed by two-way repeated-measures analysis of variance (ANOVA) followed by Tukey post hoc test using SigmaPlot software (Systat Software Inc, Chicago, IL). Other data were analyzed by one-way or two-way ANOVA followed by Tukey post hoc test. P < 0.05 was considered significant.
3 Results
3.1 Efficacy of GLT-1 down-regulation and effect on NSIA
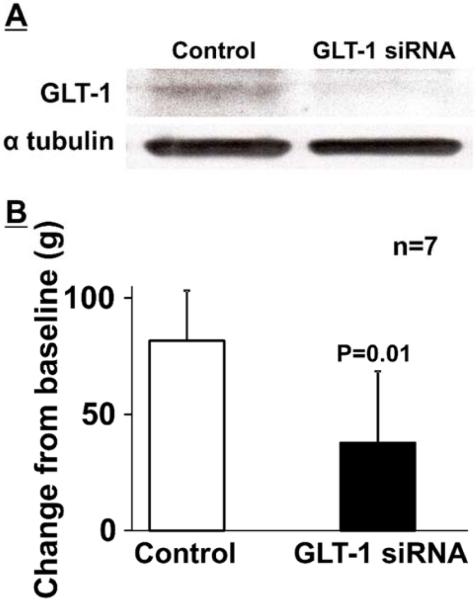
Figure 1A depicts representative immunoblotting images of GLT-1 in the right LC treated with the GLT-1 selective siRNA and non-targeting siRNA (Control). Quantitatively, GLT-1 siRNA treatment significantly reduced expression of GLT-1 in the LC (ratio to α-tubulin: 0.35 ± 0.13, n=7) compared to the control (ratio to α-tubulin: 0.62 ± 0.27, p=0.02, n=7 in each group). In these rats, mechanical withdrawal thresholds in the right hindpaw of GLT-1 siRNA treated rats (137 ± 7 g) did not differ from those of control rats (140 ± 13 g, p=0.83). A right forepaw injection of capsaicin increased withdrawal thresholds from the baseline in the right hindpaw 30 min after injection, demonstrating NSIA (Figure 1B). GLT-1 siRNA treatment significantly reduced the NSIA compared to the control (p=0.01). These results confirm our previous observation in animals with neuropathic injury [8] and suggest that down-regulation of GLT-1 in the LC impairs NSIA.
Figure 1.
(A) Representative western blotting images of GLT-1 and α-tubulin in the right LC from non-targeting siRNA (Control) and GLT-1 selective siRNA treated rats. (B) Control and GLT-1 siRNA treated rats received a subdermal injection of capsaicin (150 µg/50 µl) into the right forepaw. Withdrawal thresholds in the right hindpaw were measured prior to (baseline) and 30 min after capsaicin. Data (mean + SD) are presented as change from baseline.
3.2 Simultaneous microdialysis for LC glutamate and spinal noradrenaline
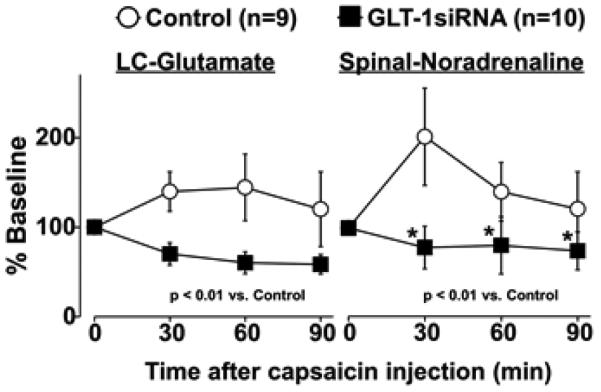
Basal glutamate concentrations in microdialysates from the right LC of GLT-1 siRNA treated rats (3.5 ± 1.6 ng/30 μl, n=10) were significantly greater than those of control rats (1.5 ± 0.7 ng/30 μl, p<0.01, n=9). Similarly, basal noradrenaline concentrations in microdialysates from the right lumbar spinal dorsal horn of GLT-1 siRNA treated rats (1.6 ± 0.5 pg/30 μl) were significantly greater than those of control rats (0.5 ± 0.2 pg/30 μl, p<0.01). These results suggest that down-regulation of GLT-1 in the LC increases basal activity of spinally projecting LC neurons.
For capsaicin-evoked glutamate release in the LC, there were significant main effects of group (F1, 51 = 50.32, p<0.01) and the group X time interaction (F3, 51 = 18.19, p<0.01), but no main effect of time (F3, 51 = 2.52, p=0.07) (Figure 2). For capsaicin-evoked noradrenaline release in the spinal cord, there were significant main effects of group (F1, 51 = 51.59, p<0.01), time (F3, 51 = 7.15, p<0.01), and the group X time interaction (F3, 51 = 12.28, p<0.01) (Figure 2B). Post hoc testing revealed that GLT-1 siRNA significantly reduced capsaicin-evoked spinal noradrenaline release compared to the control during the experiment (p<0.01), consistent with the NSIA result.
Figure 2.
Simultaneous microdialysis in the right LC and lumbar spinal dorsal horn. Changes in glutamate and noradrenaline concentrations in microdialysates from the LC and spinal cord from control or GLT-1 siRNA treated rats are presented over the time as percentage of baseline. Data are presented as mean ± SD. *p<0.01 vs Control.
3.3 LC neuronal activity
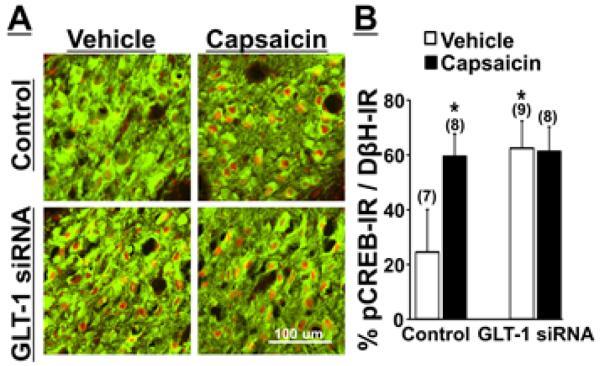
Photomicrographs in Figure 3A depict pCREB-IR in noradrenergic LC neurons, identified by DβH-IR, in GLT-1 siRNA treated or control rats with a forepaw injection of vehicle or capsaicin at the same dose used for NSIA. Quantitatively, there were significant main effects of group (F1, 28 = 23.12, p< 0.01), treatment (F1, 28 = 16.90, p< 0.01) and the group X treatment interaction (F1, 28 = 19.33, p< 0.01) (Figure 3B, n=7-9). Post hoc testing revealed that GLT-1 siRNA treatment significantly increased pCREB-IR in LC neurons compared to the control in vehicle groups (p<0.01) and that capsaicin increased pCREB-IR in LC neurons compared to vehicle in control groups (p < 0.01), whereas no such increase occurred in capsaicin injected rats compared to vehicle injected rats in GLT-1 siRNA treated groups (p=0.84), consistent with current NSIA and the microdialysis results.
Figure 3.
(A) Photomicrographs of DβH–IR (green) and pCREB-IR (red) in the right LC collected from control or GLT-1 siRNA treated rats 30 min after the right forepaw injection of vehicle or capsaicin. (B) Quantification. Data (mean + SD) are presented as the percentage of pCREB-IR in DβH-IR neurons. *p<0.01 vs Vehicle treated Control.
4. Discussion
Glutamate is the most ubiquitous excitatory neurotransmitter in the central nervous system and its regulation by glutamate transporters has been intensely investigated in the spinal cord and brain. The current study supports previous observations in rats after peripheral nerve injury which indicate a role of GLT-1 down-regulation in the LC as an important mechanism for the impaired endogenous analgesia in chronic neuropathic pain [8], and extends these observations by showing in normal rats that knock-down of GLT-1 in the LC alone is sufficient to impair pain-evoked LC activity and endogenous analgesia.
As a primary excitatory neurotransmitter in the LC, glutamate acts on AMPA receptors to increase noradrenergic neuronal activity [19]. Consistently, knock-down of GLT-1 increases basal extracellular glutamate level to increase basal noradrenergic neuronal activity in the LC, and subsequently increases basal spinal noradrenaline in normal rats. Interestingly, although stimulation of spinal α2 adrenoceptors produces antinociception in normal rats [3, 14], the current study demonstrates that increased endogenous spinal noradrenergic tone does not affect mechanical withdrawal thresholds, consistent with no antinociceptive effects of the LC activation from an intra-LC injected gabapentin in normal rats [6], and indicating there is little or no role of endogenous noradrenergic tone in physiological nociception. Increased synaptic concentrations of glutamate can activate presynaptic mGluRs to auto-inhibit glutamate release from its terminals [13]. Consistently, previous and current results showed that increased extracellular glutamate level by either peripheral nerve injury or knock-down of GLT-1 decreases capsaicin-evoked LC glutamate release and subsequently reduces evoked LC neuronal activation and spinal noradrenaline release, resulting in impaired NSIA which is reversed by an intra-LC injected mGluR antagonist [8]. These results suggest that the tonic inhibition of presynaptic glutamatergic terminals because of elevated extracellular glutamate impairs responsivity of the LC to noxious stimuli and NSIA, and that knock-down of GLT-1 in the LC alone mimics these effects of peripheral nerve injury in the LC.
In the current study, knock-down of GLT-1 in the LC increases basal activity of the LC spinal noradrenergic pathway and impairs evoked LC activity. This is consistent with the adaptive gain concept [1], supported by the previous work in animals showing that cortical responses to non-noxious sensory stimuli are modulated by basal LC activity, with reduced response in the presence of high basal activity [2]. Similar effects of basal LC activity on the role of the LC in decision making in human [7, 12] also support this concept. However, our previous study demonstrated that removal of auto-inhibition on glutamatergic terminals can further activate LC neurons with high basal activity, by showing in rats after peripheral nerve injury that blockade of presynaptic mGluRs in the LC with high basal activity reduces mechanical hypersensitivity and restores NSIA via activation of descending noradrenergic inhibition [8]. These results may suggest that basal LC activity affects in non-noxious and noxious stimuli-evoked LC activity differently. The current study used pCREB as a marker for neuronal activity and could not examine whether increased basal LC activity by increased extracellular glutamate level affects pain-evoked LC activity. Future studies should examine this relationship more directly via LC neuronal recording.
In summary, the current study demonstrates that astroglial GLT-1 is essential to normal LC function and increased extracellular glutamate by down-regulation of GLT-1 in the LC is critical to impairment of pain-evoked endogenous analgesia in rats. Since LC activity regulates physiological functions in the central nervous system, including not only pain but also cognition, sleep and level of arousal, and anxiety, knock-down of GLT-1 in the LC can be a good tool to study the role of LC on these important functions.
Highlights.
Down-regulating GLT-1 increases basal extracellular glutamate level in the LC.
Elevated basal glutamate reduces pain-evoked glutamate release in the LC.
Elevated basal glutamate increases basal but reduces pain-evoked LC activity.
Glutamate dysregulation in the LC impairs pain-evoked endogenous analgesia.
Acknowledgements
This work was supported by grants DA27690 to K.H. from the National Institutes of Health (Bethesda, Maryland).
Abbreviations
- LC
locus coeruleus
- NSIA
noxious stimulation-induced analgesia
- mGluRs
metabotropic glutamate receptors
- GLT-1
glutamate transporter-1
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest. Dr. Eisenach has served as a consultant to Adynxx, Inc. and Aerial Biopharma regarding preclinical and early phase clinical novel analgesic drug development.
References
- [1].Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- [2].Devilbiss DM, Waterhouse BD. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J Neurophysiol. 2011;105:69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duflo F, Li X, Bantel C, Pancaro C, Vincler M, Eisenach JC. Peripheral nerve injury alters the alpha2 adrenoceptor subtype activated by clonidine for analgesia. Anesthesiology. 2002;97:636–641. doi: 10.1097/00000542-200209000-00018. [DOI] [PubMed] [Google Scholar]
- [4].Ferrari LF, Gear RW, Levine JD. Attenuation of activity in an endogenous analgesia circuit by ongoing pain in the rat. J Neurosci. 2010;30:13699–13706. doi: 10.1523/JNEUROSCI.2867-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J Cogn Neurosci. 2011;23:1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- [8].Kimura M, Suto T, Morado-Urbina CE, Peters CM, Eisenach JC, Hayashida K. Impaired Pain-evoked Analgesia after Nerve Injury in Rats Reflects Altered Glutamate Regulation in the Locus Coeruleus. Anesthesiology. 2015;123:899–908. doi: 10.1097/ALN.0000000000000796. [DOI] [PubMed] [Google Scholar]
- [9].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- [10].Medrano MC, Gerrikagoitia I, Martinez-Millan L, Mendiguren A, Pineda J. Functional and morphological characterization of glutamate transporters in the rat locus coeruleus. British journal of pharmacology. 2013;169:1781–1794. doi: 10.1111/bph.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Millan MJ. Descending control of pain. Progress in neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- [12].Murphy PR, Robertson IH, Balsters JH, O'Connell G. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. R. [DOI] [PubMed] [Google Scholar]
- [13].Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117:141–161. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paqueron X, Conklin D, Eisenach JC. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology. 2003;99:199–204. doi: 10.1097/00000542-200307000-00030. [DOI] [PubMed] [Google Scholar]
- [15].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2007. [Google Scholar]
- [16].Peters CM, Hayashida K, Suto T, Houle TT, Aschenbrenner CA, Martin TJ, Eisenach JC. Individual Differences in Acute Pain-induced Endogenous Analgesia Predict Time to Resolution of Postoperative Pain in the Rat. Anesthesiology. 2015;122:895–907. doi: 10.1097/ALN.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- [18].Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- [19].Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Progress in neurobiology. 1998;56:237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- [20].Suto T, Severino AL, Eisenach JC, Hayashida K. Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology. 2014;81:95–100. doi: 10.1016/j.neuropharm.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]