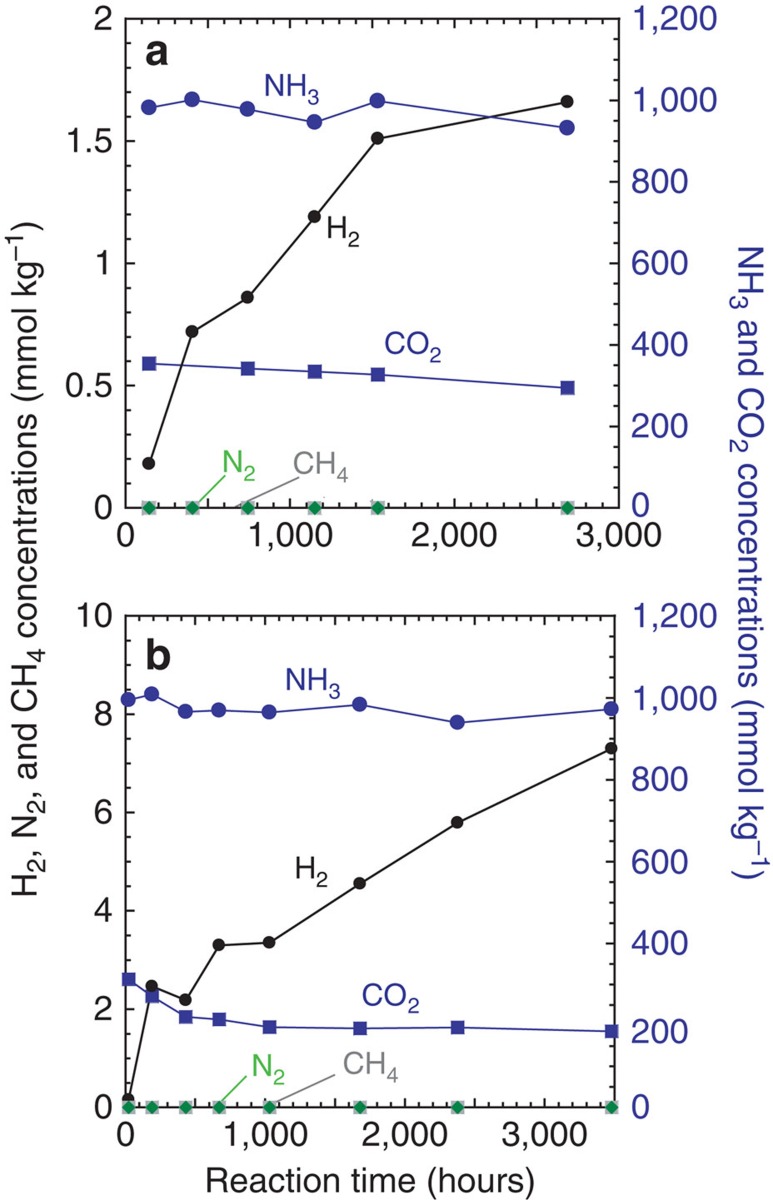
Figure 1. Variations in the concentrations of dissolved gas species.
Results of H2, N2, CH4, ΣCO2 (=CO2(aq)+CO32−+HCO3−) and ΣNH3 (=NH3(aq)+NH4+) during the experiments of (a) the opx experiment at 300 °C, and (b) the olivine experiment at 300 °C. Dissolved H2 was generated through the oxidation of Fe(II) in olivine to magnetite and serpentine, which were observed in the rocks after the experiments. The decreasing ΣCO2 is due to the formation of carbonate in the solid phase. The ΣNH3 concentrations are high and almost constant during the experiments. Our results provide no evidence for CH4 or N2 production from CO2 or NH3, respectively (CH4 production <5 μmol kg−1; N2 production <50 μmol kg−1) (see Supplementary Note 1). The experimental data are given in the Supplementary Table 2.