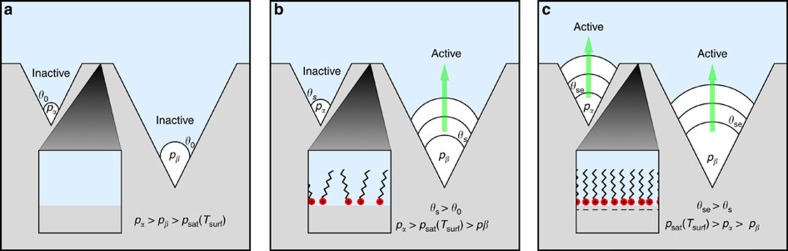
Figure 2. Nucleation behaviour with surfactants and electric potential.
A small and large conical cavity at temperature Tsurf are shown under different conditions of surfactant and electric potential. (a) When no surfactants are present, the Laplace pressure within the entrapped vapour is high due to a low contact angle. Consequently, the pressure inside both bubbles is too high for saturation conditions; thus, no evaporation occurs. (b) When surfactants are added, they adsorb to the solid–liquid interface in a tail-up configuration19, which would cause the surface to appear more hydrophobic, increasing the contact angle and lowering the Laplace pressure, which in this case is enough to cause evaporation in the larger cavity. (c) When electric potential is applied with surfactants such that they are electrostatically attracted to the surface, the number of surfactants at the solid–liquid interface increases, further increasing contact angle, which is enough to activate both nucleation sites. In all cases, the bubble in the larger cavity starts with a larger initial volume because the volume fraction of the cone occupied by vapour during the trapping (wetting) process is constant since it depends on cone angle and contact angle, which are the same for all cavities35.