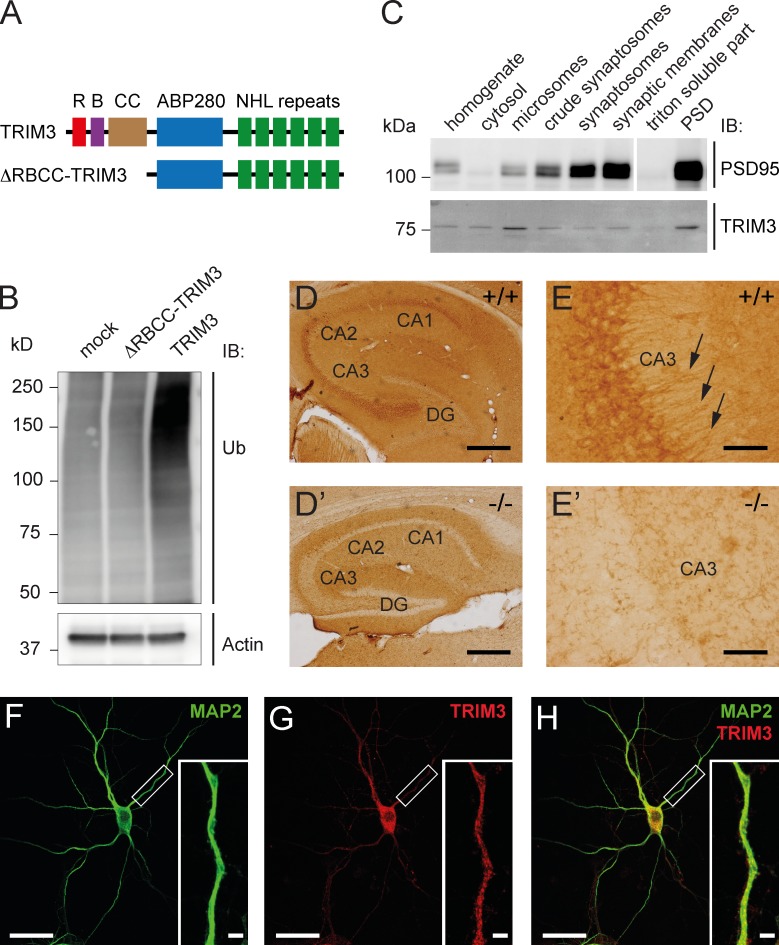
Figure 1.
TRIM3 is a dendritically localized ubiquitin ligase in the hippocampus. (A) Schematic diagram of full-length TRIM3 and ΔRBCC-TRIM3 constructs. R, RING; B, B-box; CC, coiled-coil; ABP280, actin-binding protein 280 repeat; NHL repeat, NCL-1/HT2A/LIN-41 repeat. (B) TRIM3, but not ΔRBCC-TRIM3, induces polyubiquitylation in HEK293 cells. HEK293 cell lysates were subjected to immunoblot (IB) analysis using an anti-ubiquitin (Ub) antibody. Increased polyubiquitylation is indicated by a strong increase in high-molecular-weight ubiquitin staining. Actin staining is shown as loading control. (C) Biochemical fractionation of hippocampal tissue followed by immunoblot analysis shows that TRIM3 protein is enriched in the postsynaptic density fraction and in microsomes. Synaptic enrichment is shown by PSD-95 staining of the same protein samples. (D and E) Immunohistochemistry shows that TRIM3 protein is expressed throughout the CA regions of the hippocampus, but less in the dentate gyrus (DG; D). TRIM3 protein was primarily detected in CA pyramidal cell bodies, but also in dendrites (arrows in E). Staining of hippocampal sections from Trim3−/− animals confirmed the specificity of the TRIM3 labeling (D′ and E′). Bars: (D) 400 µm; (E) 50 µm. (F–H) Immunocytochemistry revealed a punctuate staining of TRIM3 in dendrites of cultured primary hippocampal neurons. Neurons were labeled with antibodies against MAP2 (green; F) and TRIM3 (red; G). TRIM3-positive puncta are observed inside MAP2-positive dendrites (H). Bars: 25 µm; (insets) 2.5 µm.