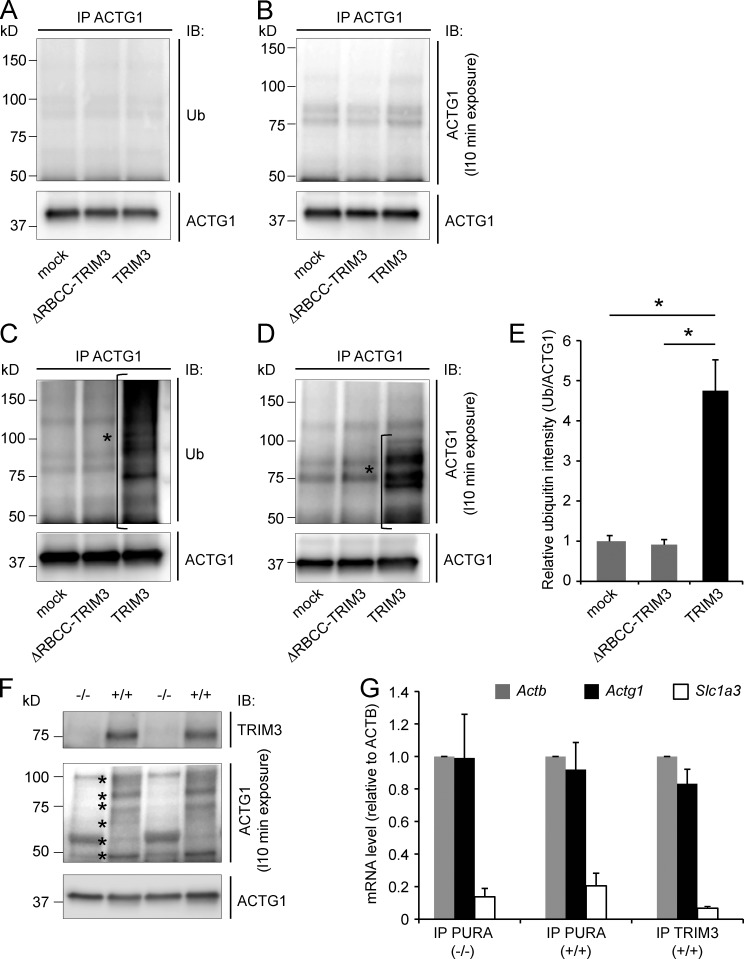
Figure 6.
TRIM3 polyubiquitylates ACTG1. (A–D) TPA stimulation induces ACTG1 polyubiquitylation by TRIM3 in HEK293 cells. HEK293 cells were transfected with TRIM3 or ΔRBCC-TRIM3. After incubation for 4 h in the presence of MG132, cells were lysed and ACTG1 was immunoprecipitated from the lysates, resolved on SDS-PAGE, and immunoblotted. Blots were first stained for polyubiquitin (A) and then stripped and restained for ACTG1 (B). Under these basal conditions, only unmodified ACTG1 was detected. However, when cells were incubated in the presence of MG132 and TPA, a significant increase in high-molecular-weight polyubiquitylated forms of ACTG1 (indicated with *) was observed specifically in TRIM3-transfected cells (C–E; means ± SEM, two-tailed t test, *, P < 0.05, n = 3 cultures per condition). (F) TPA induces ACTG1 polyubiquitylation by TRIM3 in hippocampal neurons. Cultured hippocampal neurons from wild-type and Trim3−/− mice were treated with MG132 and TPA for 4 h. Lysates were immunoblotted and stained for ACTG1. TPA induced the appearance of multiple high molecular weight (50–100 kD) bands (indicated with *) consistent with polyubiquitylation and specifically in neurons from wild-type mice and not from Trim3−/− mice. (G) Actg1 mRNA is present in TRIM3/PURA containing mRNP granules. mRNP granules were immunoprecipitated from hippocampal lysates with antibodies against TRIM3 or PURA. mRNA was isolated from immunoprecipitates, reverse transcribed into cDNA, and used for real-time quantitative PCR. Actg1 and Actb mRNA was detected at equal levels in all precipitates, whereas the negative control mRNA Slc1a3 was ∼10-fold lower detected (means ± SEM, n = 3 immunoprecipitations per condition).