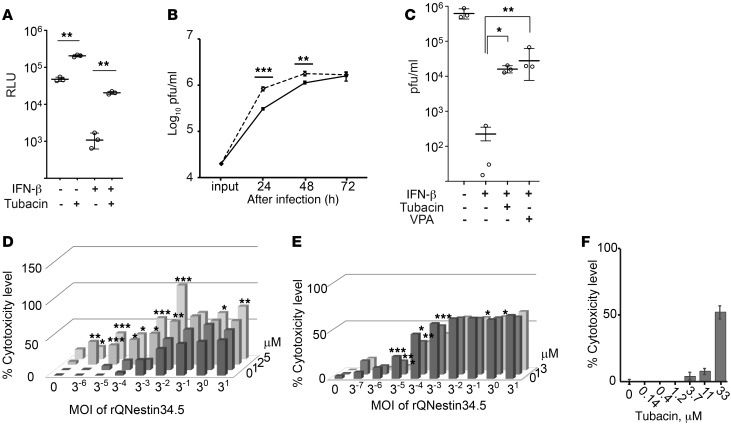
Figure 1. The HDAC6-specific inhibitor, tubacin, improves HSV-1–mediated gene expression and oHSV replication.
(A) Bioluminescence (measured as RLU) assay was performed 24 hours after infection with a replication-defective HSV-1 encoding a Fluc cDNA of U251 cells (MOI of 3). (B) Replication of rQNestin34.5 (MOI of 0.1) in tubacin-treated (dashed line) and control U251 cells (solid line). The input dose was given at 0 hours. (C) Titration of oHSV-infected (rQNestin34.5-infected) U251 cells (MOI of 0.03) in the presence of IFN-β, with and without VPA or tubacin, 3 days after infection. Doses of tubacin and IFN-β were 5 μM and 1,000 units/ml, respectively. (D) LDH cytotoxicity assay 3 days after infection of U251 cells by rQNestin34.5 in the presence of tubacin (0, 1, 2, and 5 μM; starting at 14 hours before infection). (E) LDH cytotoxicity assay 5 days after infection of U251 cells by rQNestin34.5 in the presence of CI994 (0, 1, and 3 μM; starting at 14 hours before infection). (F) LHD cytotoxicity assay of U251 cells in the presence of tubacin for 5 days. *P < 0.05, **P < 0.01, ***P < 0.001 by 1-way ANOVA test in A–C and F) and 1-way ANOVA with Turkey’s multiple comparisons tests in D and E. Error bars correspond to mean ± SD (n = 3; in A–C and n = 4; in F). Horizontal bars represent the average in A–C. Bars represent the average (n = 4; in D–F) (See also Supplemental Figure 1.).