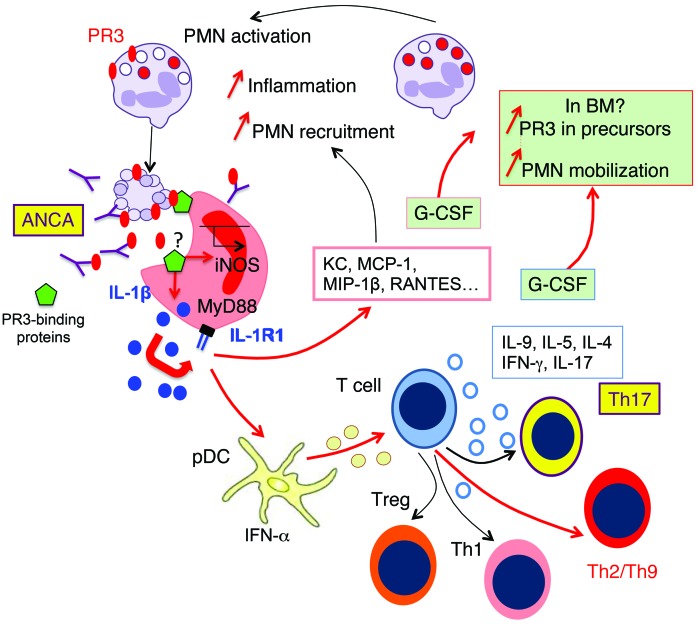
Figure 10. Apoptotic cells expressing phosphatidylserine-associated PR3 induce proinflammatory responses and favor Th9 polarization: in vivo switch to Th17 in the presence of PR3 ANCA.
Phagocytosis of apoptotic cells expressing phosphatidylserine-associated PR3 stimulates the production of inflammatory chemokines by macrophages (depicted in red) through the IL-1R1/MyD88 signaling pathway and NO production, thus promoting the recruitment of inflammatory cells expressing PR3, including neutrophils and monocytes. This process acts as an amplification loop and perpetuates inflammation. The presence of phosphatidylserine-associated PR3 on apoptotic cells generates a proinflammatory microenvironment, which facilitates the production of Th2/Th9- and Th1-activated CD4+ T cells through the interaction between pDCs and naive T cells. Importantly, the presence of anti-PR3 ANCA induces Th17 cell production. Increased G-CSF production by macrophages and by T cells may result in an increased PR3 biosynthesis and mobilization of neutrophils from BM, suggesting that PR3 participates in an autoamplification loop, which in turn sustains inflammation. Expression of phosphatidylserine-associated PR3 on apoptotic neutrophils, which prevents the resolution of inflammation, appears to be a key element in the pathogenesis of GPA. PMN, neutrophils.