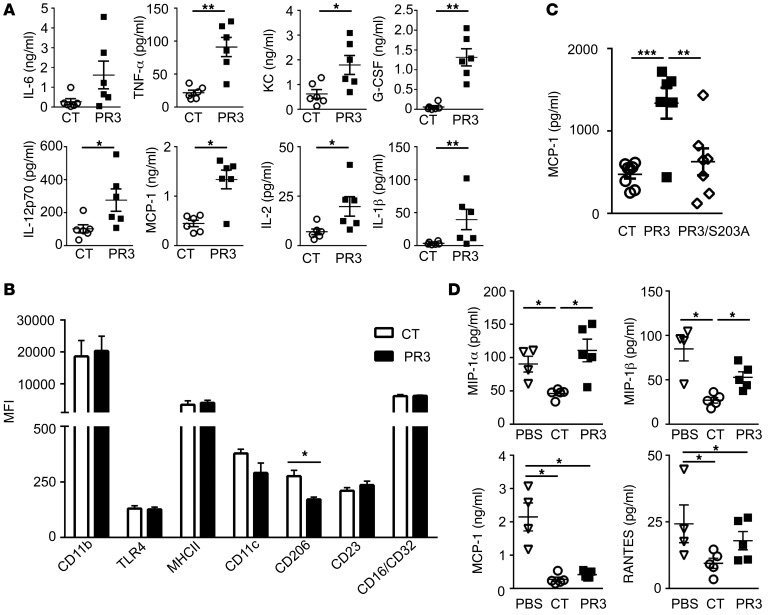
Figure 2. Membrane expression of PR3 on apoptotic cells triggered a proinflammatory response in vivo that required serine protease activity.
(A) Apoptotic control (CT) (white circles ) or PR3-expressing (black squares) cells were injected i.p. and peritoneal lavage fluid collected after 2 hours. IL-6, TNF-α, KC, G-CSF, IL-12p70, MCP-1, IL-2, and IL-1β were assessed using the proinflammatory mouse cytokine assay (n = 5 mice per group, each cytokine measured in triplicate). (B) Using flow cytometry, polarization of F4/80+ macrophages was examined by assessing expression of various cell-surface markers, including CD11b, TLR4, MHCII, CD11c, CD206, CD23, and CD16/CD32 (n = 4 mice per group, each marker measured in duplicate). (C) Apoptotic control (n = 8), PR3- (n = 6), or PR3/S203A-expressing cells (n = 8, white diamonds) were injected i.p. in mice for 2 hours as in A and MCP-1 measured in duplicates in the peritoneal lavage by ELISA. (D) PBS (white triangles), apoptotic control, or PR3-expressing cells were injected i.p. 72 hours after peritonitis was induced with thioglycolate. The peritoneal lavage fluid was collected after a further 24 hours and concentrations of MIP-1α, MIP-1β, MCP-1, and RANTES determined (n = 5 mice per group, each cytokine measured in triplicate). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Significant differences between groups were determined by multicomparison ANOVA (A–C) or Mann-Whitney U test (D).