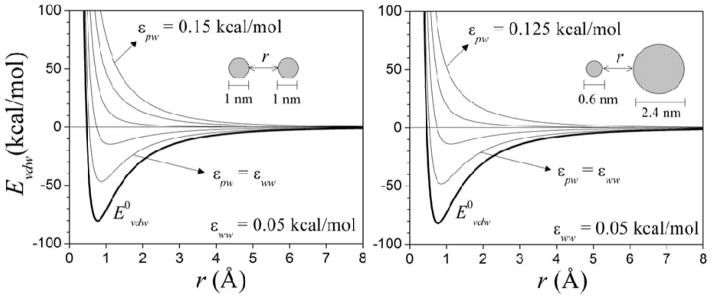
Figure 3.
van der Waals interaction energy Evdw (eq 4) between two spheres in water, as a function of the distance r between their surfaces, and for different values εww and εpw (eq 6). The spheres are composed of identical atoms distributed uniformly on a cubic lattice with a constant of 1.4 Å. Lennard-Jones parameters are εij = 0.15 kcal/mol and σij = 2.5 Å (LB mixing rules used). The energies at equilibrium are similar (within ~2 kcal/mol) in both systems for the same values of εww and εpw. The direct potentials (E0vdw; for εww = εpw = 0) corresponding to the uncorrected interactions are also shown.