Abstract
Ciliary neurotrophic factor (CNTF) is neuroprotective against multiple pathologic conditions including metabolic impairment, but the mechanisms are still unclear. To delineate CNTF effects on brain energy homeostasis, we performed a multimodal imaging study, combining in vivo proton magnetic resonance spectroscopy, high-performance liquid chromatography analysis, and in situ glutamate imaging by chemical exchange saturation transfer. Unexpectedly, we found that CNTF expression through lentiviral gene transfer in the rat striatum significantly decreased the levels of neuronal metabolites (N-acetyl-aspartate, N-acetyl-aspartyl-glutamate, and glutamate). This preclinical study shows that CNTF remodels brain metabolism, and suggests that decreased levels of neuronal metabolites may occur in the absence of neuronal dysfunction.
Keywords: brain imaging, glutamate, magnetic resonance, N-acetyl-aspartate, reactive astrocytes, striatal metabolism
Introduction
The cytokine ciliary neurotrophic factor (CNTF) exhibits well-established neuroprotective effects against a large variety of pathologic conditions, including excitotoxicity and metabolic impairment.1, 2 It has been tested in clinical trials for Huntington's disease,3 amyotrophic lateral sclerosis,4 and diseases of the retina.5 However, the mechanisms involved in CNTF neuroprotection remain largely uncharacterized.
Ciliary neurotrophic factor is known to directly activate catabolic pathways in peripheral organs such as the liver, muscle, and adipose tissues, and to regulate food intake and energy expenditure in the hypothalamus.6 Besides these metabolic effects, CNTF is a potent activator of astrocytes. We previously reported that astrocyte activation by CNTF alters their metabolic profile, leading to protective effects on neurons exposed to glycolytic inhibition.7
Here, we aimed to further delineate CNTF metabolic effects on the intact brain. We performed a multimodal imaging study combining proton magnetic resonance spectroscopy (1H-MRS), high-performance liquid chromatography (HPLC) analysis and brain mapping of glutamate (Glu) with Chemical Exchange Saturation Transfer (gluCEST) on rats injected with a lentiviral vector encoding CNTF.
Materials and methods
Injection of Lentiviral Vectors
We used self-inactivated lentiviral vectors that encode either the human CNTF gene (‘lenti-CNTF') or the β-galactosidase gene (‘lenti-LacZ').8
Two-month-old male Sprague Dawley rats (Charles River, France) were injected with lentiviral vectors as described previously,9 and analyzed between 1.5 and 3 months after injection (CNTF effects being stable for at least 6 months8). To avoid bias associated with lateralization, both combinations of injections (lenti-LacZ in the left striatum and lenti-CNTF in the right striatum, or the opposite) were performed.
Housing and experiments were performed in strict accordance with the European Community regulations (Directive 2010-63/EEC) and French regulations (Code Rural R214/87-130). Experimental procedures were approved by a local ethics committee registered by the French Research Ministry (committee #44, approval #10-057).
Proton Magnetic Resonance Spectroscopy
Proton magnetic resonance spectroscopy (1H-MRS) experiments were performed on a horizontal 7 T Agilent scanner (Palo Alto, CA, USA). We used a volume coil for radiofrequency transmission and a quadrature surface coil for reception.
T2-weighted images were acquired with a 2D fast spin-echo sequence, and were then used to position two voxels of 4 × 3.4 × 3 mm3 in each striatum for 1H-MRS analysis (Figure 1B). Excellent shimming (9 to 11 Hz) was achieved in these voxels. A LASER (Localization by Adiabatic Selective Refocusing) sequence with echo time/repetition time=25/5,000 ms was used for 1H-MRS acquisition (384 repetitions for metabolite spectrum). Pulse bandwidth of the hyperbolic secant pulses in LASER was equal to 10 kHz, ensuring less than 6% chemical shift localization artifact over the spectral range of the metabolites of interest (2 to 4 ppm).
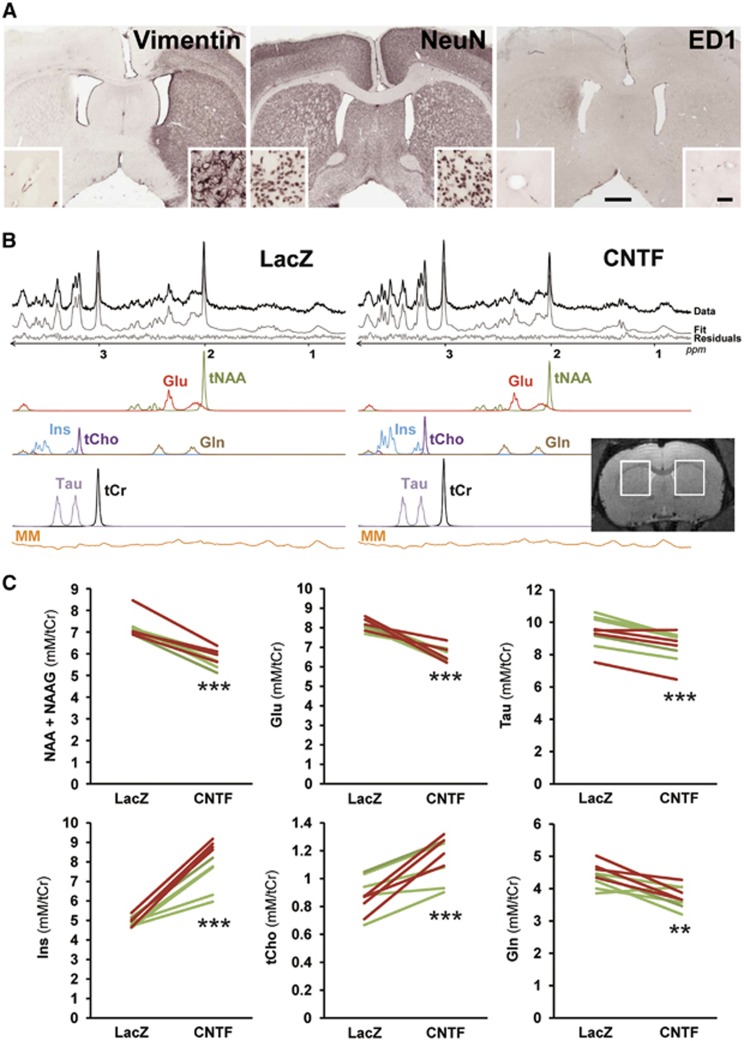
Figure 1.
Ciliary neurotrophic factor (CNTF) remodels striatal metabolite concentrations as measured by proton magnetic resonance spectroscopy (1H-MRS). (A) Representative images of immunostaining for vimentin, NeuN, and ED1/CD68. Vimentin is strongly induced in the lenti-CNTF injected striatum (right), but not in the lenti-LacZ striatum (left). At higher magnification, multiple reactive astrocytes are observed. There is no difference in the density or the morphology of neuronal cell bodies between the two striata. There is no positive staining for ED1/CD68, a selective marker of reactive microglia. Scale bar: 1 mm (large panels), 50 μm (small panels). (B) Representative in vivo 1H-MRS spectra obtained from the two striata, 2 months after lentiviral injection. Analysis by LCModel allows spectra decomposition into (1) neuronal metabolites (total NAA (tNAA=N-Acetyl-Aspartate (NAA)+ N-Acetyl-Aspartyl-Glutamate (NAAG)) and Glutamate (Glu)); (2) glial metabolites (Inositol (Ins), total Choline (tCho) and Glutamine (Gln)) (3) other metabolites (Taurine (Tau), total creatine (tCr), and macromolecules (MM)). Inset, representative T2-weighted image showing the position of the voxel (total volume=40.8 μL). There is no visible difference in T2 contrast between striata. (C) Metabolite concentrations measured in both voxels. Green lines correspond to rats injected with lenti-LacZ in the left striatum and lenti-CNTF in the right striatum; red lines correspond to rats injected with lenti-CNTF in the left striatum and lenti-LacZ in the right striatum. N=9; **P<0.01, ***P<0.001, paired t-test.
Metabolite concentrations were quantified with LCModel.10 A macromolecule spectrum was acquired by metabolite nulling and included in LCModel basis set. Seven metabolites were reliably quantified (Cramér-Rao lower bounds ⩽5%). Metabolite concentrations were normalized to total creatine, set at 8 mmol/L.
Chemical Exchange Saturation Transfer Imaging of Glutamate
The gluCEST imaging was performed on a horizontal 11.7 T Bruker scanner with a volume coil for radiofrequency transmission and a quadrature surface coil for reception (Bruker, Ettlinger, Germany). The ‘Mapshim' routine was applied in a voxel encompassing the slice of interest and a B1 map was also acquired to correct for inhomogeneities of the saturation pulse. A gluCEST image centered on the injection sites was acquired with a 2D fast spin-echo sequence preceded by a frequency-selective continuous-wave saturation pulse (300 × 300 μm2 in-plane resolution, 1 mm slice thickness) with echo time/repetition time=6/5,000 ms, 10 echoes and effective echo time=30 ms. The saturation pulse was applied during 1 second with a 5-μT B1 amplitude. Two images were acquired with saturation pulse applied either at +3 ppm (Msat(+3 ppm), the center of CEST peak for Glu) or at a symmetrical frequency relative to bulk water (Msat(−3 ppm)). The gluCEST contrast was calculated pixel by pixel, as a percentage, by the equation: 100 × (Msat(−3 ppm)−Msat(+3ppm))/Msat(−3 ppm).
High-Performance Liquid Chromatography Analysis
Six rats were killed with a lethal dose of pentobarbital 4 months after lentiviral vector injection. Each striatum was rapidly dissected out, weighted, and frozen in isopentane. Samples were stored at −80°C until HPLC analysis coupled with UV detection of N-Acetyl-Aspartate (NAA) and N-Acetyl-Aspartyl-Glutamate (NAAG), according to a protocol adapted from.11
ELISA Measurement of Human Ciliary Neurotrophic Factor
The striatum of five lenti-CNTF rats and four sham-operated rats was rapidly dissected out on ice, homogenized in PBS with 1% Triton X-100 and protease inhibitors (Roche, Basel, Switzerland). After centrifugation (20,000 g, 15 minutes, 4°C), the supernatant was collected and after fivefold dilution, analyzed by ELISA with the Quantikine ELISA Human CNTF immunoassay kit in reference to standards, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).12
Immunohistochemistry
Immunohistochemistry was performed on the brain of paraformaldehyde-perfused rats as described previously,8 with the following antibodies: ED1/CD68 (1:500; Serotec, Raleigh, NC, USA), NeuN (1:2,000; Millipore, Billerica, MA, USA), and vimentin (1:2,000; Calbiochem, La Jolla, CA).
Statistical Analysis
Results are expressed as mean±SEM. Paired t-tests were performed with Statview. N indicates the number of animals. No blinding was performed but rats were injected randomly with lenti-CNTF in either the left or right striatum.
Results
Lentiviral Vector-Mediated Expression of Ciliary Neurotrophic Factor in the Rat Striatum
We first measured the levels of human CNTF by ELISA, 5 weeks after infection with lenti-CNTF in the rat striatum. Ciliary neurotrophic factor levels were 2.39±0.53 ng/mg total protein in lenti-CNTF injected rats, while it was below 3.10−4 ng/mg total protein in sham-operated rats.
To further evidence CNTF production in the rat striatum, we performed immunostaining for vimentin, a marker of reactive astrocytes known to be induced by CNTF.8, 9 Astrocytes overexpressed vimentin in a large part of the striatum injected with lenti-CNTF (28.47±4.80 mm3, Figure 1A).
We also checked that lentiviral injection or transgene expression had no detrimental effect on striatal cells. There was no detectable change in NeuN staining and no immunoreactivity for ED1/CD68, a marker for activated microglia (Figure 1A), as already reported.8, 9
Ciliary Neurotrophic Factor Induces Multiple Changes in Metabolite Concentrations
Rats injected with lenti-LacZ in one striatum and lenti-CNTF in the contralateral striatum were imaged using a 7 T magnet, 1.5 to 3 months after infection. There was no detectable change in T2-weighted signal in the striatum for any of the nine rats studied (Figure 1B). Proton magnetic resonance spectroscopy was performed on two voxels positioned over each striatum (Figure 1B). High-quality spectra were acquired (Figure 1B), allowing the reliable quantification of seven metabolites, using total creatine as a reference.
There was a significant increase in the concentrations of two metabolites enriched in glial cells: myo-inositol (Ins, +61±7%, P<0.001) and total choline (glycerophosphocholine+phosphocholine+choline, tCho, +33±7%, P<0.001), in the CNTF striatum compared with LacZ (Figure 1C). On the contrary, the astrocyte metabolite glutamine (Gln) was decreased in the CNTF striatum (−14±3%, P<0.01). Surprisingly, CNTF also decreased the levels of the neuronal metabolites NAA+NAAG (tNAA, −19±2%, P<0.001) and glutamate (Glu, −18±2%, P<0.001, Figure 1C). Taurine (Tau) concentrations were also significantly decreased by CNTF (−9±1%, P<0.001). The same pattern was observed independently of the side of lenti-CNTF injection (Figure 1C), ruling out a possible bias due to voxel position, chemical shift artifact, or intrinsic differences between hemispheres. The same metabolic profile was observed in three independent cohorts of rats.
The Decrease in N-Acetyl-Aspartate and N-Acetyl-Aspartyl Glutamate Concentrations Is Confirmed by High-Performance Liquid Chromatography Analysis
To measure NAA concentrations by an alternative method, we performed HPLC analysis coupled with UV detection on striatal samples. This method allows the separate measurement of NAAG, which is otherwise combined with the main NAA peak on the 1H-MRS spectrum.
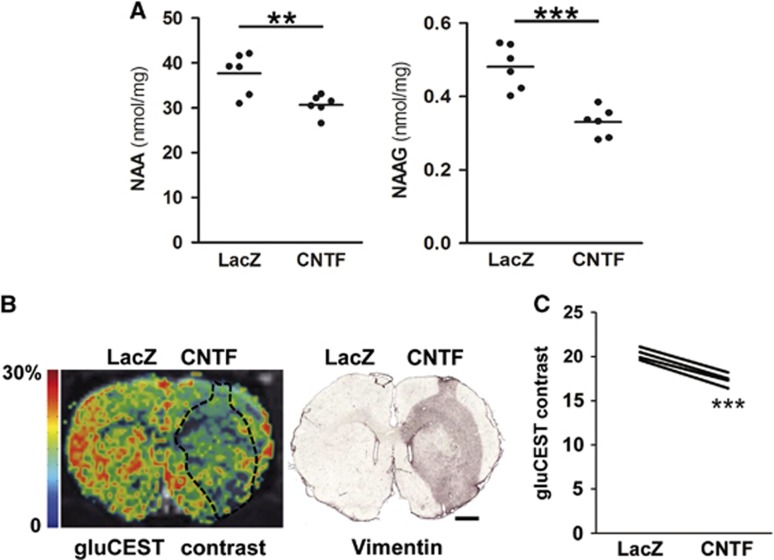
N-Acetyl-Aspartate levels were significantly decreased by 18±3% in the CNTF striatum compared with the LacZ striatum (P<0.01, Figure 2A), which is very similar to the difference measured by 1H-MRS. There was also an important decrease in NAAG levels with CNTF (−31±3%, P<0.001, Figure 2A).
Figure 2.
High-performance liquid chromatography (HPLC) analysis confirms decreased NAA and NAAG levels with ciliary neurotrophic factor (CNTF) and chemical exchange saturation transfer of glutamate (gluCEST) imaging reveals lower Glu levels in the striatum displaying CNTF-activated astrocytes. (A) Quantification of NAA and NAAG by HPLC coupled with UV detection on postmortem samples. Data are expressed as nmol/mg of tissue (wet weight). N=6; **P<0.01, ***P<0.001, paired t-test. (B) A representative gluCEST image exhibiting a lower gluCEST contrast in the white matter, e.g., in the corpus callosum and anterior commissure, as described previously.13 GluCEST contrast is decreased in the hemisphere injected with lenti-CNTF. The region with vimentin+ reactive astrocytes (black dots) closely matches the region exhibiting lower gluCEST contrast. (C) Quantification of the average gluCEST contrast in voxels similar to the ones used for proton magnetic resonance spectroscopy (1H-MRS). N=4, ***P<0.001, paired t-test. Scale bar: 1 mm.
Chemical Exchange Saturation Transfer of Glutamate Imaging Reveals a Decrease in Glutamate Levels that Matches the Volume of Astrocyte Activation
To confirm the decrease in Glu levels with CNTF and to map its spatial extension in vivo, we took advantage of the recently developed NMR imaging technique called gluCEST.13 The gluCEST contrast is correlated with Glu concentrations, allowing in vivo Glu mapping with good spatial and temporal resolution.13 A lower gluCEST contrast was observed in the CNTF striatum (Figure 2B). The brain area displaying vimentin-positive reactive astrocytes matched precisely the zone exhibiting lower gluCEST contrast (Figure 2B). To compare gluCEST with 1H-MRS results, we calculated the average gluCEST contrast in the same striatal voxels analyzed by 1H-MRS. A significant reduction in gluCEST contrast was measured in the voxel positioned over the CNTF striatum compared with the LacZ striatum (−14±1%, P<0.001, Figure 2C), which is consistent with 1H-MRS measurements.
Discussion
Our study shows that CNTF triggers complex changes in striatal metabolism, affecting metabolites enriched in astrocytes as well as those enriched in neurons. The significant decrease in Gln levels shows that CNTF does not globally increase the concentrations of glial metabolites, but rather modulates the relative abundance of specific brain metabolites. By alternating the side of lentiviral vector injections, we ruled out possible bias due to spectroscopic localization or lateralization. In addition, 1H-MRS results were validated by two alternative methods. The decrease in NAA+NAAG concentrations was confirmed ex vivo by HPLC analysis, providing an independent measurement of these two metabolites. The decrease in Glu levels was also observed by gluCEST imaging and closely matched the volume displaying CNTF-activated astrocytes.
Ciliary neurotrophic factor is a neurotrophic cytokine with demonstrated protective effects against multiple brain insults including excitotoxicity1, 12 and metabolic impairment.2, 7 Ciliary neurotrophic factor neuroprotective effects were reproduced in nonhuman primates, opening the path to clinical trials for amyotrophic lateral sclerosis,4 diseases of the retina5 and Huntington's disease.3 Therefore, it is rather unexpected that CNTF reduces neuronal metabolite levels in the rat striatum. Indeed, decreased tNAA levels are classically interpreted as reflecting neuronal death or at least dysfunction in 1H-MRS studies.14 However, using the same strategy to overexpress CNTF in the rat striatum, we previously showed that CNTF does not alter the expression of many neuronal proteins,8 does not modify the spontaneous electrophysiologic activity of striatal neurons,15 and confers significant neuroprotection against excitotoxicity.15 This was consistent with two independent studies based on lentiviral vectors,12, 16 in which intrastriatalal CNTF levels were in the ng/mg protein range, as measured in the present study. Other delivery modes (adenoviral gene transfer2 and encapsulated cells releasing CNTF17) confirmed the protective effects of CNTF against striatal lesions. The levels of CNTF achieved with these different strategies are not directly comparable to our ELISA measurement. Encapsulated cells are known to produce CNTF less efficiently due to their low survival rate after implantation.18 This may explain the limited beneficial effects observed in a phase I clinical trial for HD patients,3 although this strategy was very efficient in primates.19 Considering the strong evidence for CNTF neuroprotective effects in the striatum, our results show that decreased tNAA and Glu levels may occur independently of neuronal dysfunction, and may instead be associated with significant neuroprotective effects.
Overall, CNTF induces a complex reorganization of striatal metabolism. Accordingly, we previously showed that CNTF activates the AMP kinase, a master regulator of energy metabolism,7 and that CNTF alters the metabolic profile of astrocytes.7 Given the importance of energy homeostasis for proper neuronal function, it is tempting to speculate that such metabolic plasticity may participate in CNTF neuroprotective effects, as demonstrated in an in vitro model of metabolic impairment.7
The molecular mechanisms mediating CNTF effects on neuronal metabolites remain to be determined; they may be direct or indirect. Indeed, both NAA and Glu metabolism involve complex interactions between neurons, astrocytes but also oligodendrocytes.20 The facts that (1) CNTF acts primarily on astrocytes and (2) there is a strong spatial correlation between reactive astrocytes and decreased Glu levels observed by gluCEST, suggest that astrocytes may be involved in the remodeling of striatal metabolism.
Overall, we show that the neurotrophic cytokine CNTF induces significant metabolic plasticity in brain cells. Such metabolic signature would be classically interpreted as neuronal dysfunction or death in 1H-MRS clinical studies. Instead, our results raise the intriguing possibility that it may indicate ongoing compensatory mechanisms.
The authors declare no conflict of interest.
Footnotes
Author Contributions
MACS, JF, YB, LBH, MG, AB, GA, DH, JV, and CE performed experiments, MACS, JF, JV, and CE designed research, analyzed data and wrote the manuscript, EB, GB, and PH contributed reagents/materials and edited the manuscript.
The authors thank N Dufour, C Joséphine, Pr. N Déglon, and Dr A Bémelmans for viral vector production. We are grateful to P Gipchtein and Dr C Jan for their help with immunostainings. The authors thank Dr V Lebon and C Marchadour for valuable inputs on NMR acquisition.
This work was supported by the grants ANR 2010-JCJC-1402-1 (to CE), by a grant ‘Investissement d'Avenir–ANR-2011-INBS-0011'–NeurATRIS (to PH), CEA and CNRS.
References
- 1Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, Chu Y et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature 1997; 386: 395–399. [DOI] [PubMed] [Google Scholar]
- 2Mittoux V, Ouary S, Monville C, Lisovoski F, Poyot T, Conde F et al. Corticostriatopallidal neuroprotection by adenovirus-mediated ciliary neurotrophic factor gene transfer in a rat model of progressive striatal degeneration. J Neurosci 2002; 22: 4478–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Bloch J, Bachoud-Levi AC, Deglon N, Lefaucheur JP, Winkel L, Palfi S et al. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther 2004; 15: 968–975. [DOI] [PubMed] [Google Scholar]
- 4Aebischer P, Schluep M, Deglon N, Joseph JM, Hirt L, Heyd B et al. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med 1996; 2: 696–699. [DOI] [PubMed] [Google Scholar]
- 5Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA 2006; 103: 3896–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Matthews VB, Febbraio MA. CNTF: a target therapeutic for obesity-related metabolic disease? J Mol Med 2008; 86: 353–361. [DOI] [PubMed] [Google Scholar]
- 7Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M et al. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci 2007; 27: 7094–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C et al. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci 2006; 26: 5978–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci 2012; 32: 10809–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679. [DOI] [PubMed] [Google Scholar]
- 11Battistuta J, Bjartmar C, Trapp BD. Postmortem degradation of N-acetyl aspartate and N-acetyl aspartylglutamate: an HPLC analysis of different rat CNS regions. Neurochem Res 2001; 26: 695–702. [DOI] [PubMed] [Google Scholar]
- 12de Almeida LP, Zala D, Aebischer P, Deglon N. Neuroprotective effect of a CNTF-expressing lentiviral vector in the quinolinic acid rat model of Huntington's disease. Neurobiol Dis 2001; 8: 433–446. [DOI] [PubMed] [Google Scholar]
- 13Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H et al. Magnetic resonance imaging of glutamate. Nat Med 2012; 18: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007; 81: 89–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Beurrier C, Faideau M, Bennouar KE, Escartin C, Kerkerian-Le Goff L, Bonvento G et al. Ciliary neurotrophic factor protects striatal neurons against excitotoxicity by enhancing glial glutamate uptake. PLoS One 2010; 5: e8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Régulier E, Pereira de Almeida L, Sommer B, Aebischer P, Deglon N. Dose-dependent neuroprotective effect of ciliary neurotrophic factor delivered via tetracycline-regulated lentiviral vectors in the quinolinic acid rat model of Huntington's disease. Hum Gene Ther 2002; 13: 1981–1990. [DOI] [PubMed] [Google Scholar]
- 17Emerich DF, Lindner MD, Winn SR, Chen EY, Frydel BR, Kordower JH. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington's disease. J Neurosci 1996; 16: 5168–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Emerich DF, Thanos CG. Intracompartmental delivery of CNTF as therapy for Huntington's disease and retinitis pigmentosa. Curr Gene Ther 2006; 6: 147–159. [DOI] [PubMed] [Google Scholar]
- 19Mittoux V, Joseph JM, Conde F, Palfi S, Dautry C, Poyot T et al. Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington's disease. Hum Gene Ther 2000; 11: 1177–1187. [DOI] [PubMed] [Google Scholar]
- 20Moffett JR, Arun P, Ariyannur PS, Namboodiri AM. N-Acetylaspartate reductions in brain injury: impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front Neuroenergetics 2013; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]