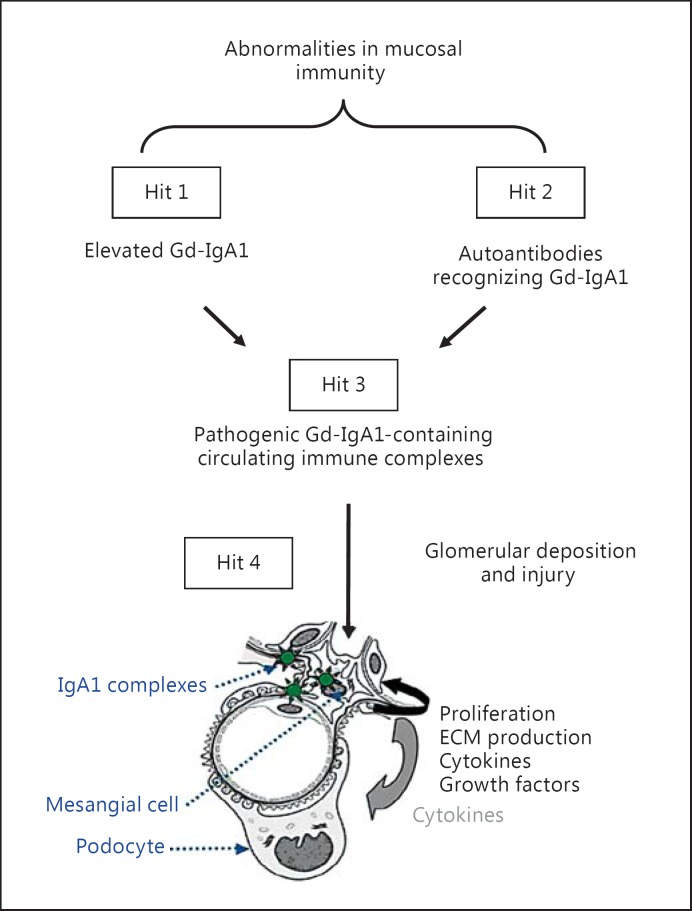
Fig. 1.
Hypothesis on the pathogenesis of IgA nephropathy. Synthesis of IgA1 with some O-glycans deficient in galactose (autoantigen) is elevated. Gd-IgA1 is present in the circulation at increased levels (hit 1). This immunoglobulin is recognized by unique circulating anti-glycan autoantibodies (hit 2) [13,14,20]. This process results in the formation of pathogenic IgA1-containing circulating immune complexes (hit 3), some of which deposit in the glomeruli and induce renal injury (hit 4) [20]. Upstream factors are likely involved in abnormal mucosal immune responses characteristic for patients with IgA nephropathy [3]. An alternative hypothesis has been proposed to suggest that aberrantly glycosylated IgA1 accumulates in the mesangium as lanthanic deposits that are later bound by newly appearing autoantibodies, resulting in the in situ formation of immune complexes [92]. All hits and additional factors may be genetically regulated; 15 distinct genetic loci have been identified by genome-wide association studies to be associated with the risk of IgA nephropathy [7,38]. It is certainly possible that other loci may influence the mechanisms of disease or the clinical expression of IgA nephropathy. ECM = Extracellular matrix.