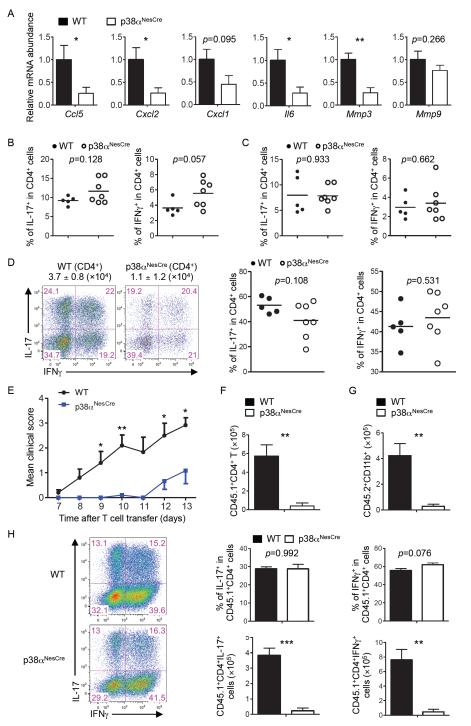
Fig. 3. Ablation of p38α in CNS-resident cells inhibits expression of genes encoding chemokines and inflammatory factors and represses TH17 cell–induced EAE.
(A) Spinal cord cells from WT and p38αNesCre EAE mice on day 11 after immunization were subjected to real-time PCR analysis to determine the relative abundances of the indicated mRNAs. Data are means ± SEM of five to seven mice per group. (B to D) Cells were isolated from (B) the spleens, (C) DLNs, and (D) spinal cords of MOG-immunized WT and p38αNesCre mice (on day 11), stimulated with PMA and ionomycin in vitro, and then analyzed by flow cytometry to determine the percentages of cells positive for IL-17 and IFN-γ among CD4+ T cells. Data are means ± SEM of five to seven mice per group. (D) The total numbers of CD4+ T cells that infiltrated the spinal cords are indicated above the flow cytometry plots. (E) EAE disease course of WT and p38αNesCre mice (CD45.2+) that received in vitro–derived TH17 cells from CD45.1 mice. Data are means ± SEM of 10 mice per group. (F and G) Analysis of the numbers of (F) donor-derived CD4+ (CD45.1+) T cells and (G) host-derived (CD45.2+CD11b+) myeloid cells isolated from the spinal cords of WT and p38αNesCre mice on day 11 after adoptive transfer of cells. Data are means ± SEM of four mice per group. (H) Left: Cells were isolated from the spinal cords of recipient WT and p38αNesCre mice on day 11 after transfer, stimulated with PMA and ionomycin in vitro, and then analyzed by flow cytometry to determine the percentages of cells positive for IL-17 and IFN-γ among donor-derived CD4+ (CD45.1+) T cells. Right: Data are means ± SEM of four mice per group. *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of two to three independent experiments.